
How are the following compounds obtained from benzoic acid?
a.) Ethyl benzoate
b.) Benzyl alcohol
c.) Benzene
Answer
602.1k+ views
Hint: . Benzoic acid: It is an organic compound which has a chemical formula of ${ C }_{ 6 }{ H }_{ 5 }{ COOH }$. In this, the carboxyl group is attached to the benzene ring. Benzene can be obtained from benzoic acid in the presence of sodium hydroxide and soda-lime. Ethyl benzoate can be obtained from benzoic acid by using the esterification reaction. Benzyl alcohol can be obtained from benzoic acid by using the hydrogenation reaction.
Complete answer:
A. Ethyl benzoate: When benzoic acid is esterified with ethanol in the presence of an acid catalyst then ethyl benzoate will be produced.
The following reaction will take place:
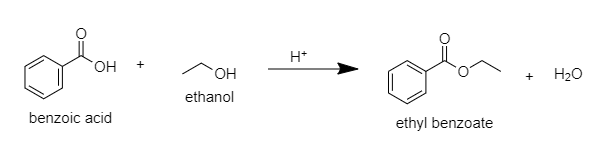
B.Benzyl alcohol: When benzoic acid is reduced with lithium aluminum hydride then benzaldehyde will be formed and then again it is reduced then benzyl alcohol will be formed.
The following reaction will take place:
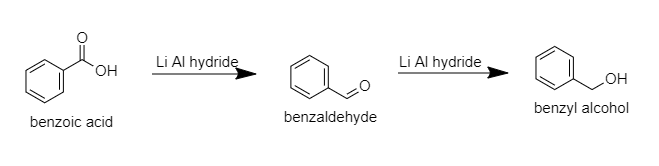
C. Benzene: When benzoic acid is heated with soda lime, decarboxylation will take place and benzene will be formed.

Additional Information: Esterification is the reaction in which a carboxylic acid reacts with an alcohol in the presence of concentrated sulphuric acid to form an ester. Esters are basic in natural science and organic materials and regularly have a trademark charming, fruity smell.
Soda Lime: It is a mixture of calcium oxide and sodium hydroxide which is commonly known as soda-lime, (CaO/NaOH). It is used as a drying agent and carbon dioxide absorbent and as a decarboxylating agent.
Decarboxylation (i.e., elimination of ${ CO }_{ 2 }$ ) of sodium salts of carboxylic acids on heating with soda lime (a mixture of NaOH and CaO) gives alkane.
Note: The possibility to make a mistake is that in the formation of benzene from benzoic acid, a decarboxylation reaction (removal of carbon dioxide) occurs, not decarbonylation which is the removal of CO.
Ethyl benzoate can be obtained from benzoic acid by esterification, not acid hydrolysis. Acid hydrolysis is simply the reverse of esterification.
Benzene can be obtained from benzoic acid using soda-lime which is a drying agent. But don’t add too much to the drying agent as it will reduce the yield of the product.
Complete answer:
A. Ethyl benzoate: When benzoic acid is esterified with ethanol in the presence of an acid catalyst then ethyl benzoate will be produced.
The following reaction will take place:

B.Benzyl alcohol: When benzoic acid is reduced with lithium aluminum hydride then benzaldehyde will be formed and then again it is reduced then benzyl alcohol will be formed.
The following reaction will take place:

C. Benzene: When benzoic acid is heated with soda lime, decarboxylation will take place and benzene will be formed.

Additional Information: Esterification is the reaction in which a carboxylic acid reacts with an alcohol in the presence of concentrated sulphuric acid to form an ester. Esters are basic in natural science and organic materials and regularly have a trademark charming, fruity smell.
Soda Lime: It is a mixture of calcium oxide and sodium hydroxide which is commonly known as soda-lime, (CaO/NaOH). It is used as a drying agent and carbon dioxide absorbent and as a decarboxylating agent.
Decarboxylation (i.e., elimination of ${ CO }_{ 2 }$ ) of sodium salts of carboxylic acids on heating with soda lime (a mixture of NaOH and CaO) gives alkane.
Note: The possibility to make a mistake is that in the formation of benzene from benzoic acid, a decarboxylation reaction (removal of carbon dioxide) occurs, not decarbonylation which is the removal of CO.
Ethyl benzoate can be obtained from benzoic acid by esterification, not acid hydrolysis. Acid hydrolysis is simply the reverse of esterification.
Benzene can be obtained from benzoic acid using soda-lime which is a drying agent. But don’t add too much to the drying agent as it will reduce the yield of the product.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




