
What are the conjugate acid-base pairs in the following reaction?
\[{{H}_{3}}P{{O}_{4}}(aq)+{{H}_{2}}O(l)\to {{H}_{2}}PO_{4}^{-}(aq)+{{H}_{3}}{{O}^{+}}(aq)\]
A) ${{H}_{3}}P{{O}_{4}}(aq)/{{H}_{2}}O(l)$
B) ${{H}_{3}}P{{O}_{4}}(aq)/{{H}_{2}}PO_{4}^{-}(aq)$
C) ${{H}_{2}}O(l)/{{H}_{2}}PO_{4}^{-}(aq)$
D) ${{H}_{2}}O(l)/{{H}_{2}}O(l)$
Answer
573.9k+ views
Hint: This question is based on Bronsted-Lowry theory of acid and base. According to this compound that gives off ${{H}^{+}}$ ion in solution is considered as acid and compound that accepts ${{H}^{+}}$ in solution is considered as base. After giving off proton species that form is the conjugate base of the acid and after accepting proton species that form is the conjugate acid of the base.
Complete step by step solution:
${{H}_{3}}P{{O}_{4}}$ is known as orthophosphoric acid and it is dibasic.
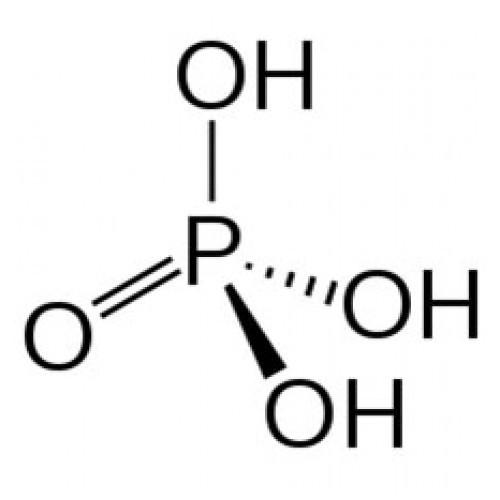
Both the hydrogens attached to oxygen atoms directly are the acidic hydrogens. In water orthophosphoric acid has the tendency to lose them.
- With water, we know that it is amphoteric, in the presence of base it acts like an acid and in presence of acid it behaves like a base.
- As mentioned in the question, we are having the solution of orthophosphoric acid. So here water will act like a base and accept protons from acid.
\[{{H}_{3}}P{{O}_{4}}(aq)+{{H}_{2}}O(l)\to {{H}_{2}}PO_{4}^{-}(aq)+{{H}_{3}}{{O}^{+}}(aq)\]
- When an acid loses a proton the specie it forms has the capacity to accept ${{H}^{+}}$because it is having an atom that has lone pair so ${{H}_{2}}PO_{4}^{-}$ can act like a base. Because it has formed from ${{H}_{3}}P{{O}_{4}}$ acid when it has lost a proton, which is why ${{H}_{2}}PO_{4}^{-}$ will be the conjugate base of ${{H}_{3}}P{{O}_{4}}$.
- So conjugate acid-base pair over here is ${{H}_{3}}P{{O}_{4}}(aq)/{{H}_{2}}PO_{4}^{-}(aq)$
- On the other hand in above reaction water is accepting the proton and forming ${{H}_{3}}{{O}^{+}}$ ion and this ${{H}_{3}}{{O}^{+}}$tendency to lose proton and get back into the stable molecule like water and because this ${{H}_{3}}{{O}^{+}}$ has formed from water when it accepted proton, which is why ${{H}_{3}}{{O}^{+}}$ will be the conjugate acid of ${{H}_{2}}O$
- So conjugate acid-base pair over here is ${{H}_{2}}O(l)/{{H}_{3}}{{O}^{+}}$
Out of both these conjugate acid-base pairs only one is mentioned in Option (B).
So, the correct answer is “Option B”.
Additional Information:
Bronsted-Lowry theory explains why compounds like ammonia act as a base and also explains why compounds like carboxylic acid show acidic behavior in alcoholic solution, which wasn’t explained by Arrhenius theory.
Note: knowledge of conjugate acid-base pairs help us to identify the strength of acids and bases. If acid is strong its conjugate base will be weak and if acid is weak its conjugate base will be strong and vice versa. For example ${{C}_{2}}{{H}_{5}}OH$ is a weak acid that’s why its conjugate base \[{{C}_{2}}{{H}_{5}}{{O}^{-}}\] is a very strong base, in fact it is stronger base than hydroxide ion.
Complete step by step solution:
${{H}_{3}}P{{O}_{4}}$ is known as orthophosphoric acid and it is dibasic.

Both the hydrogens attached to oxygen atoms directly are the acidic hydrogens. In water orthophosphoric acid has the tendency to lose them.
- With water, we know that it is amphoteric, in the presence of base it acts like an acid and in presence of acid it behaves like a base.
- As mentioned in the question, we are having the solution of orthophosphoric acid. So here water will act like a base and accept protons from acid.
\[{{H}_{3}}P{{O}_{4}}(aq)+{{H}_{2}}O(l)\to {{H}_{2}}PO_{4}^{-}(aq)+{{H}_{3}}{{O}^{+}}(aq)\]
- When an acid loses a proton the specie it forms has the capacity to accept ${{H}^{+}}$because it is having an atom that has lone pair so ${{H}_{2}}PO_{4}^{-}$ can act like a base. Because it has formed from ${{H}_{3}}P{{O}_{4}}$ acid when it has lost a proton, which is why ${{H}_{2}}PO_{4}^{-}$ will be the conjugate base of ${{H}_{3}}P{{O}_{4}}$.
- So conjugate acid-base pair over here is ${{H}_{3}}P{{O}_{4}}(aq)/{{H}_{2}}PO_{4}^{-}(aq)$
- On the other hand in above reaction water is accepting the proton and forming ${{H}_{3}}{{O}^{+}}$ ion and this ${{H}_{3}}{{O}^{+}}$tendency to lose proton and get back into the stable molecule like water and because this ${{H}_{3}}{{O}^{+}}$ has formed from water when it accepted proton, which is why ${{H}_{3}}{{O}^{+}}$ will be the conjugate acid of ${{H}_{2}}O$
- So conjugate acid-base pair over here is ${{H}_{2}}O(l)/{{H}_{3}}{{O}^{+}}$
Out of both these conjugate acid-base pairs only one is mentioned in Option (B).
So, the correct answer is “Option B”.
Additional Information:
Bronsted-Lowry theory explains why compounds like ammonia act as a base and also explains why compounds like carboxylic acid show acidic behavior in alcoholic solution, which wasn’t explained by Arrhenius theory.
Note: knowledge of conjugate acid-base pairs help us to identify the strength of acids and bases. If acid is strong its conjugate base will be weak and if acid is weak its conjugate base will be strong and vice versa. For example ${{C}_{2}}{{H}_{5}}OH$ is a weak acid that’s why its conjugate base \[{{C}_{2}}{{H}_{5}}{{O}^{-}}\] is a very strong base, in fact it is stronger base than hydroxide ion.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




