
What are the bond angles of $ P{F_3}C{l_2} $ ?
Answer
518.4k+ views
Hint :The bond angle generally depends on the number of lone electron pairs, size and electronegativity of the central atom and also the size of atoms surrounding it. Note that the molecule $ P{F_3}C{l_2} $ has a trigonal bipyramidal geometry.
Complete Step By Step Answer:
Phosphorus atoms have fifteen electrons with a total of five valence electrons. Three of these electrons are involved in bond formation with fluorine atoms and two electrons are involved in the bond formation with chlorine atoms. So, the hybridization of $ P{F_3}C{l_2} $ is $ s{p^3}d $ . So, the geometry of the molecule $ P{F_3}C{l_2} $ is trigonal bipyramidal with asymmetric charge distribution on the central atom. The fluorine atom is more electronegative and it occupies the axial position. Chlorine being less electronegative will occupy the equatorial position. In the $ P{F_3}C{l_2} $ molecule, the two chlorine atoms take up the planar position. The dipole moments of the two apex fluorine atoms cancel each other. Now, there will be a dipole moment in the triangular plane. The two $ P - Cl $ dipole moments are not cancelled out by one $ P - F $ dipole moment. This will leave a net dipole moment in the plane, which makes $ P{F_3}C{l_2} $ a polar molecule. For a $ E{X_5} $ structure, there will be $ 3 \times \angle X - E - X = {120^ \circ } $ for the equatorial $ X $ ligands and there will be $ 1 \times \angle X - E - X = {180^ \circ } $ . The structure of the molecule $ P{F_3}C{l_2} $ is given below.
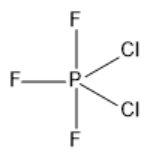
Note :
Remember that the bond angle of a molecule depends mainly on the number of lone pairs and the electronegativity of the central atom. Note that more electronegative atoms occupy the axial position in a molecule which has trigonal bipyramidal geometry.
Complete Step By Step Answer:
Phosphorus atoms have fifteen electrons with a total of five valence electrons. Three of these electrons are involved in bond formation with fluorine atoms and two electrons are involved in the bond formation with chlorine atoms. So, the hybridization of $ P{F_3}C{l_2} $ is $ s{p^3}d $ . So, the geometry of the molecule $ P{F_3}C{l_2} $ is trigonal bipyramidal with asymmetric charge distribution on the central atom. The fluorine atom is more electronegative and it occupies the axial position. Chlorine being less electronegative will occupy the equatorial position. In the $ P{F_3}C{l_2} $ molecule, the two chlorine atoms take up the planar position. The dipole moments of the two apex fluorine atoms cancel each other. Now, there will be a dipole moment in the triangular plane. The two $ P - Cl $ dipole moments are not cancelled out by one $ P - F $ dipole moment. This will leave a net dipole moment in the plane, which makes $ P{F_3}C{l_2} $ a polar molecule. For a $ E{X_5} $ structure, there will be $ 3 \times \angle X - E - X = {120^ \circ } $ for the equatorial $ X $ ligands and there will be $ 1 \times \angle X - E - X = {180^ \circ } $ . The structure of the molecule $ P{F_3}C{l_2} $ is given below.

Note :
Remember that the bond angle of a molecule depends mainly on the number of lone pairs and the electronegativity of the central atom. Note that more electronegative atoms occupy the axial position in a molecule which has trigonal bipyramidal geometry.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




