
What are the bond angles of a molecule with trigonal bipyramidal geometry?
Answer
519.3k+ views
Hint: Bond angle of any molecule is defined as the angle between two adjacent carbon atoms. The atoms having trigonal bipyramidal geometry consist of 2 bond angles. These bond angles and the geometry of the molecule are a result of the valence electrons of the bonded atoms distributed as bond and lone pairs.
Complete answer:
The shape of any covalently bonded molecule is described using the VSEPR theory that suggests that a shape of a molecule is dependent on the valence pair of electrons in the atoms of that molecule. These valence electrons are distributed in the form of bond pairs and lone pairs. The interactions of these pairs are in the order, lone pair – lone pair > lone pair – bond pair > bond pair – bond pair. This states that the bond angles are dependent on the shape and repulsions of the bonds and lone pairs.
The molecule that has trigonal bipyramidal geometry consists of an atom having 5 atoms surrounded by a central atom. So, the bond pairs are distributed so that 2 bonds acquire the opposite sides on top and bottom called axial bonds and 3 bonds are at the plane called the equatorial bonds.
The axial bonds in the molecule have the angle of $90{}^\circ $ while; the equatorial bonds that are present beside each other have an angle of $120{}^\circ $. This happens as the axial bonds are surrounded by 3 equatorial bonds while the equatorial bonds are surrounded by 2 bonds adjacent to each of them.
Example of trigonal bipyramidal geometry is $PC{{l}_{5}}$and is as follows:
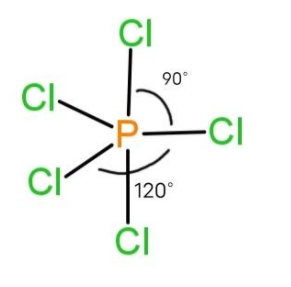
So, the bond angles in the molecule having trigonal bipyramidal geometry are $90{}^\circ $ axial bonds and $120{}^\circ $ equatorial bonds.
Note:
The VSEPR theory is able to identify shapes and geometry of various molecules through their valence electron distribution. There may be instances that a molecule can contain bond pairs along with lone pairs, so due to lone pairs the repulsion becomes maximized and the shape becomes distorted and affects the bond angle.
Complete answer:
The shape of any covalently bonded molecule is described using the VSEPR theory that suggests that a shape of a molecule is dependent on the valence pair of electrons in the atoms of that molecule. These valence electrons are distributed in the form of bond pairs and lone pairs. The interactions of these pairs are in the order, lone pair – lone pair > lone pair – bond pair > bond pair – bond pair. This states that the bond angles are dependent on the shape and repulsions of the bonds and lone pairs.
The molecule that has trigonal bipyramidal geometry consists of an atom having 5 atoms surrounded by a central atom. So, the bond pairs are distributed so that 2 bonds acquire the opposite sides on top and bottom called axial bonds and 3 bonds are at the plane called the equatorial bonds.
The axial bonds in the molecule have the angle of $90{}^\circ $ while; the equatorial bonds that are present beside each other have an angle of $120{}^\circ $. This happens as the axial bonds are surrounded by 3 equatorial bonds while the equatorial bonds are surrounded by 2 bonds adjacent to each of them.
Example of trigonal bipyramidal geometry is $PC{{l}_{5}}$and is as follows:

So, the bond angles in the molecule having trigonal bipyramidal geometry are $90{}^\circ $ axial bonds and $120{}^\circ $ equatorial bonds.
Note:
The VSEPR theory is able to identify shapes and geometry of various molecules through their valence electron distribution. There may be instances that a molecule can contain bond pairs along with lone pairs, so due to lone pairs the repulsion becomes maximized and the shape becomes distorted and affects the bond angle.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




