
How are the aromatic rings represented? Why?
Answer
554.1k+ views
Hint:To answer this question, you must know about aromaticity. Aromatic compounds are those compounds which are cyclic with conjugated double bonds. They must be planar and must follow Huckel’s rule. According to it, for a cyclic compound to be an aromatic compound it must contain $\left( {4n + 2} \right)\,\pi $electrons where n may be any whole number.
The representation of aromatic rings is done by keeping resonance in mind.
Complete step-by-step answer:We all know that different resonating structures can be drawn for an aromatic compound. They all are theoretical structures; the compound actually exists as a resonance hybrid of all the structures. We should always remember that the hybrid is always more stable than all the contributing structures.
Aromatic rings are represented by a circle inside the ring. The circle represents that all the bonds are equivalent.
Actually, the pi-electron charge in benzene and other aromatic compounds is not confined to the two carbon atoms as in alkenes which also contains a double bond. It is spread over a greater area which leads to the delocalization of the pi-electron charge.
Taking the example of benzene,
We all know that there are three alternate double bonds in benzene. They can be represented by two resonating structures but the actual structure or the resonance or the resonance hybrid is the one with a circle inside the ring.
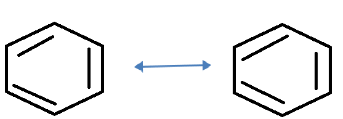
The two resonating structures of benzene is shown in this figure.
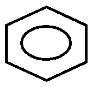
Note:It has to be noted that there are no distinct single or double bonds within the aromatic rings. The delocalization of the ring makes each count as one and a half bond between the carbons. The circle inside the aromatic rings is used to show that all the carbon-carbon bonds have the same bond-length somewhere between single and double bonds.
The representation of aromatic rings is done by keeping resonance in mind.
Complete step-by-step answer:We all know that different resonating structures can be drawn for an aromatic compound. They all are theoretical structures; the compound actually exists as a resonance hybrid of all the structures. We should always remember that the hybrid is always more stable than all the contributing structures.
Aromatic rings are represented by a circle inside the ring. The circle represents that all the bonds are equivalent.
Actually, the pi-electron charge in benzene and other aromatic compounds is not confined to the two carbon atoms as in alkenes which also contains a double bond. It is spread over a greater area which leads to the delocalization of the pi-electron charge.
Taking the example of benzene,
We all know that there are three alternate double bonds in benzene. They can be represented by two resonating structures but the actual structure or the resonance or the resonance hybrid is the one with a circle inside the ring.

The two resonating structures of benzene is shown in this figure.

Note:It has to be noted that there are no distinct single or double bonds within the aromatic rings. The delocalization of the ring makes each count as one and a half bond between the carbons. The circle inside the aromatic rings is used to show that all the carbon-carbon bonds have the same bond-length somewhere between single and double bonds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




