
How are s orbitals different from p orbitals?
Answer
565.2k+ views
Hint:To solve this question first we have to understand the term orbitals. Orbitals are 3 dimensional space around the nucleus where the probability of finding an electron is maximum.
Complete answer:
Difference between s and p orbitals is mentioned below-
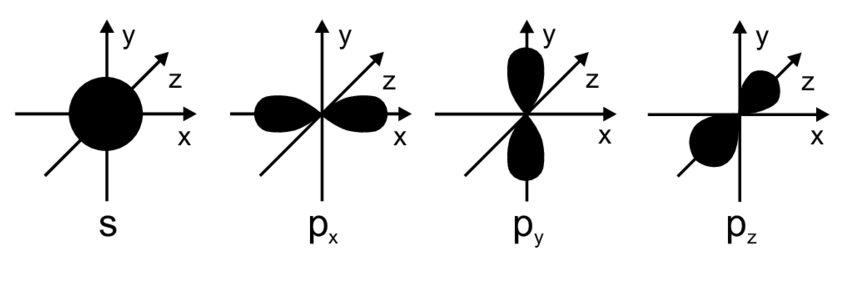
The shape of s and p orbitals is shown below:
Note:
Do not get confused between the term orbit and orbitals. Both orbit and orbitals have different meanings as we know that orbitals are 3 dimensional space around the nucleus where the probability of finding an electron is maximum and orbits are the well-defined circular paths in which electrons revolve.
Complete answer:
Difference between s and p orbitals is mentioned below-
| S orbitals | P orbitals |
| The s orbitals are atomic orbitals and the shape of s orbital is spherical. | The p orbital is also an atomic orbital and the shape of p orbital is dumbbell shape. |
| S orbitals have the lowest energy levels. | The energy levels of p orbitals are higher as compared to that of p orbitals. |
| There are no angular nodes in s orbitals. | Angular nodes are present in the p orbitals. |
| The maximum number of electrons which can be present in the s orbital is 2. | The maximum number of electrons which can be present in the p orbital is 6. |
| There are no sub orbitals present in the s orbital. | P orbitals have 3 sub orbitals. |
| The value of angular momentum quantum number for s orbitals is 0. | The value of angular momentum quantum number for s orbitals is 1. |
| There are no lobed present in the s orbitals. | There are lobes present in the p orbital. |
The shape of s and p orbitals is shown below:

Note:
Do not get confused between the term orbit and orbitals. Both orbit and orbitals have different meanings as we know that orbitals are 3 dimensional space around the nucleus where the probability of finding an electron is maximum and orbits are the well-defined circular paths in which electrons revolve.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




