
What are ‘fuel cells’? Write cathode and anode reaction in a fuel cell.
Answer
576k+ views
Hint: Fuel cell, as the name suggests, is the cell which uses fuel to generate energy. It is an electrochemical cell, consisting of two metal rods, called electrodes, dipped in an electrolyte solution. At the cathode, reduction reaction occurs, and at the anode, oxidation reaction occurs.
Complete Solution :
Fuel cells are a kind of voltaic cells which convert chemical energy to electrical energy. Unlike other voltaic cells, it can be run indefinitely provided with continuous supply of fuel. The fuel can be \[{{\text{H}}_{2}}\], CO, \[\text{C}{{\text{H}}_{4}}\], etc. Most common fuel cells use \[{{\text{H}}_{2}}\] as a fuel.
Let us study the hydrogen-oxygen fuel cell in detail to know the reaction taking place at anode and cathode. The main chemical reaction responsible for producing electrical energy is combustion of hydrogen gas. For that we shall refer to the typical diagram of fuel cells given below:
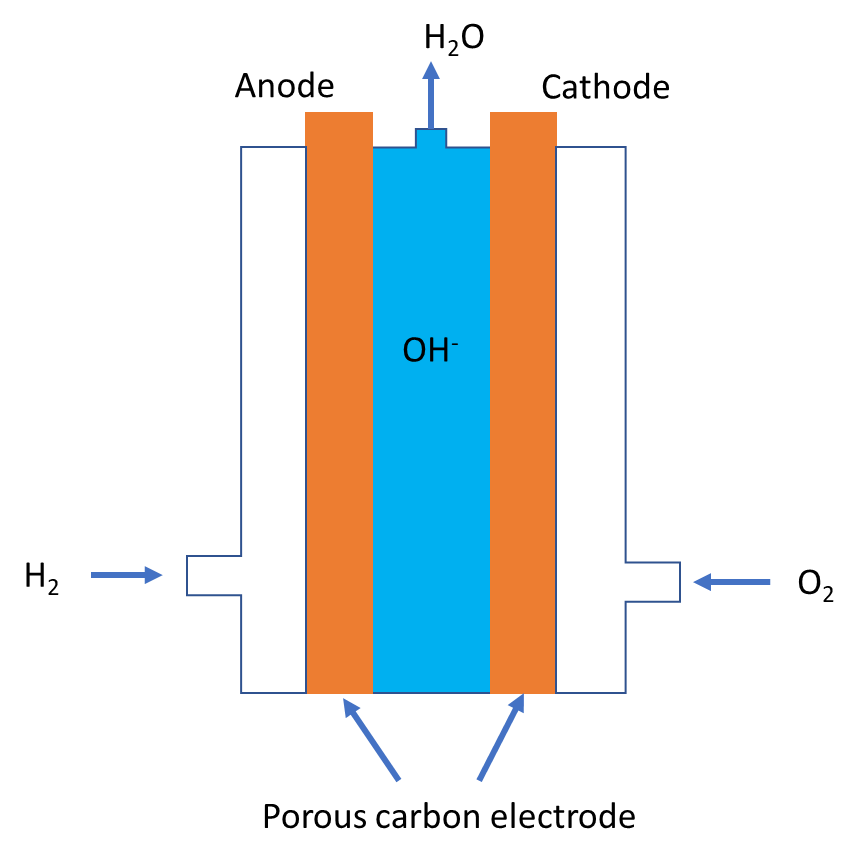
- It contains two porous carbon electrodes, which can pass fuel gas and oxygen through it and conduct electricity. Sodium hydroxide or potassium hydroxide is used as electrolyte. Hydrogen gas is supplied at anode and oxygen is supplied at cathode. The net chemical reaction of the fuel cell is given below.
\[\begin{align}
& \text{2}{{\text{H}}_{\text{2}}}\left( \text{g} \right)\text{+2}{{\text{O}}^{\text{2-}}}\to \text{2}{{\text{H}}_{\text{2}}}\text{O}\left( \text{l} \right)\text{ + 4}{{\text{e}}^{\text{-}}}\text{ }\!\![\!\!\text{ reaction at anode }\!\!]\!\!\text{ } \\
& {{\text{O}}_{\text{2}}}\left( \text{g} \right)\text{+ 4}{{\text{e}}^{\text{-}}}\to \text{2}{{\text{O}}^{\text{2-}}}\text{ }\!\![\!\!\text{ reaction at cathode }\!\!]\!\!\text{ } \\
& \overline{\text{2}{{\text{H}}_{\text{2}}}\left( \text{g} \right)\text{+}{{\text{O}}_{\text{2}}}\left( \text{g} \right)\to \text{2}{{\text{H}}_{\text{2}}}\text{O}\left( \text{l} \right)\text{ }} \\
\end{align}\]
The electrons produced at anode travel to cathode and do electrical work. It is possible only when the circuit is closed or connected to a load.
Note: There are different kinds of fuel cells, which can be explained here, fuel for these cells can be any such as CO, Methane, methanol. Accordingly, there is some modification in the setup of the fuel cell.
- Fuel cells have many merits against normal voltaic cells. Such as:
1. High efficiency,
2. Continuous source of energy, and
3. Greener approach.
Due to such merits, fuel cells are used as a source of energy generation in space stations.
Complete Solution :
Fuel cells are a kind of voltaic cells which convert chemical energy to electrical energy. Unlike other voltaic cells, it can be run indefinitely provided with continuous supply of fuel. The fuel can be \[{{\text{H}}_{2}}\], CO, \[\text{C}{{\text{H}}_{4}}\], etc. Most common fuel cells use \[{{\text{H}}_{2}}\] as a fuel.
Let us study the hydrogen-oxygen fuel cell in detail to know the reaction taking place at anode and cathode. The main chemical reaction responsible for producing electrical energy is combustion of hydrogen gas. For that we shall refer to the typical diagram of fuel cells given below:

- It contains two porous carbon electrodes, which can pass fuel gas and oxygen through it and conduct electricity. Sodium hydroxide or potassium hydroxide is used as electrolyte. Hydrogen gas is supplied at anode and oxygen is supplied at cathode. The net chemical reaction of the fuel cell is given below.
\[\begin{align}
& \text{2}{{\text{H}}_{\text{2}}}\left( \text{g} \right)\text{+2}{{\text{O}}^{\text{2-}}}\to \text{2}{{\text{H}}_{\text{2}}}\text{O}\left( \text{l} \right)\text{ + 4}{{\text{e}}^{\text{-}}}\text{ }\!\![\!\!\text{ reaction at anode }\!\!]\!\!\text{ } \\
& {{\text{O}}_{\text{2}}}\left( \text{g} \right)\text{+ 4}{{\text{e}}^{\text{-}}}\to \text{2}{{\text{O}}^{\text{2-}}}\text{ }\!\![\!\!\text{ reaction at cathode }\!\!]\!\!\text{ } \\
& \overline{\text{2}{{\text{H}}_{\text{2}}}\left( \text{g} \right)\text{+}{{\text{O}}_{\text{2}}}\left( \text{g} \right)\to \text{2}{{\text{H}}_{\text{2}}}\text{O}\left( \text{l} \right)\text{ }} \\
\end{align}\]
The electrons produced at anode travel to cathode and do electrical work. It is possible only when the circuit is closed or connected to a load.
Note: There are different kinds of fuel cells, which can be explained here, fuel for these cells can be any such as CO, Methane, methanol. Accordingly, there is some modification in the setup of the fuel cell.
- Fuel cells have many merits against normal voltaic cells. Such as:
1. High efficiency,
2. Continuous source of energy, and
3. Greener approach.
Due to such merits, fuel cells are used as a source of energy generation in space stations.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

A solution of a substance X is used for white washing class 11 chemistry CBSE

Differentiate between calcination and roasting class 11 chemistry CBSE




