
What are alkyl halides? How is n-propyl bromide prepared from propylene?
Answer
596.7k+ views
Hint: Hydrogens present in hydrocarbons can be replaced to form different classes of hydrocarbons.Use of peroxide in the reaction, leads to the formation of less substituted product. Synthesis of n-propyl bromide from propylene is an addition reaction.
Complete step by step answer:
Alkyl halides are hydrocarbons in which one or more than one hydrogen atom is replaced by halogen (chlorine, fluorine, iodine, bromine)atoms. These are also known as halogeno alkanes. Depending on the degree of substitution at the carbon atom carrying the halogen, alkyl halides are classified into primary, secondary and tertiary alkyl halides.
Propylene is an alkene hydrocarbon containing three carbon atoms, and a double bond. N-propyl bromide is synthesized by propylene by the use of HBr in presence of peroxide. Peroxide is used to get a less substituted alkyl halide.
Reaction of propylene with HBr is an addition reaction in which bromine and hydrogen are added over the doubly bonded carbon atoms. In presence of peroxide, hydrogen is added on more substituted carbon atom and bromine atom is added on less substituted carbon atom. This mechanism proceeds through Anti-markovnikov's addition.
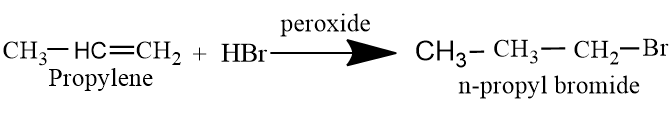
In presence of peroxide, reaction proceeds through a free radical mechanism. Free radical forms as an intermediate.During the reaction, there will be formation of two intermediates, one with primary free radical and other with secondary free radical. Since secondary free radical is more stable than primary free radical ,intermediate with secondary free radical gives the end product in major.
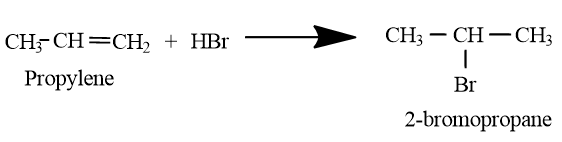
Note: In absence peroxide, reaction would have proceed through formation of carbocation and the resulting product would be 2-bromo propane, which is an isomer of n-propyl bromide.
Complete step by step answer:
Alkyl halides are hydrocarbons in which one or more than one hydrogen atom is replaced by halogen (chlorine, fluorine, iodine, bromine)atoms. These are also known as halogeno alkanes. Depending on the degree of substitution at the carbon atom carrying the halogen, alkyl halides are classified into primary, secondary and tertiary alkyl halides.
Propylene is an alkene hydrocarbon containing three carbon atoms, and a double bond. N-propyl bromide is synthesized by propylene by the use of HBr in presence of peroxide. Peroxide is used to get a less substituted alkyl halide.
Reaction of propylene with HBr is an addition reaction in which bromine and hydrogen are added over the doubly bonded carbon atoms. In presence of peroxide, hydrogen is added on more substituted carbon atom and bromine atom is added on less substituted carbon atom. This mechanism proceeds through Anti-markovnikov's addition.

In presence of peroxide, reaction proceeds through a free radical mechanism. Free radical forms as an intermediate.During the reaction, there will be formation of two intermediates, one with primary free radical and other with secondary free radical. Since secondary free radical is more stable than primary free radical ,intermediate with secondary free radical gives the end product in major.
Note: In absence peroxide, reaction would have proceed through formation of carbocation and the resulting product would be 2-bromo propane, which is an isomer of n-propyl bromide.

Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




