
How are \[1 - \] nitro propane, \[2 - \]Nitro propane and \[2 - \]methyl-\[2 - \]-nitropropane distinguished from each other using the nitrous acid?
Answer
523.2k+ views
Hint: \[2 - \]Nitropropane is a synthetic colourless liquid, flammable in nature and slightly soluble in water whereas greatly soluble in aromatic compounds like ether, hydrocarbons, esters, ketones. Its molecular formula is\[{C_3}{H_7}N{O_2}\].
Complete answer:
Now let’s move to each option one by one to see their reaction with nitrous acid, as they react with nitrous acid to produce coloured compounds.
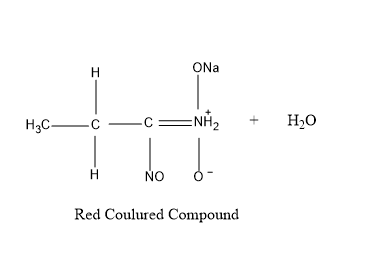
So , first taking \[1 - \] nitropropane – It reacts with nitrous acid to produce blue colored intermediate compound nitroso nitroalkanes , which further dissolves in alkali \[NaOH\] to produce red colored compound as shown in figure given below .
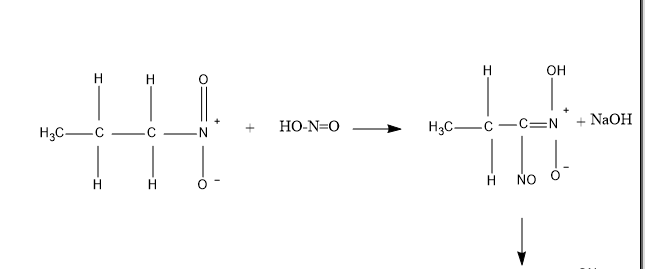
Now, talking about \[2 - \]Nitropropane – It also reacts with nitrous acid to produce blue colored intermediate compound nitroso nitroalkanes. But further it does not dissolve in alkali \[NaOH\] , because of the absence of \[\alpha - H\], (alpha hydrogen) .
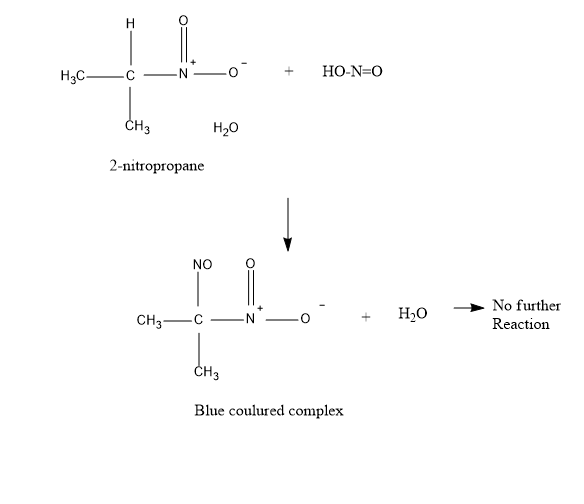
Coming to \[2 - \]methyl-\[2 - \]-nitropropane, it does not react even with nitrous acid to produce blue colored intermediate compound nitroso nitroalkanes because of the absence of\[\alpha - H\], (alpha hydrogen).
So, here we are clear about the way to distinguish between \[1 - \] nitropropane, \[2 - \]Nitropropane and \[2 - \]methyl-\[2 - \]-nitropropane.
Note:
\[1 - \] nitropropane is used as starting material for the formation of other compounds especially in paint industries. \[2 - \] Nitro propane is used as a solvent or additive in inks, varnishes, polymers. It is used as a solvent and also used in pharmaceutical companies for the formation of compounds like chlorphentermine , teclozan , phentermine . Basically they have a great use in chemical industries also especially in paint based industries .
Complete answer:
Now let’s move to each option one by one to see their reaction with nitrous acid, as they react with nitrous acid to produce coloured compounds.
So , first taking \[1 - \] nitropropane – It reacts with nitrous acid to produce blue colored intermediate compound nitroso nitroalkanes , which further dissolves in alkali \[NaOH\] to produce red colored compound as shown in figure given below .


Now, talking about \[2 - \]Nitropropane – It also reacts with nitrous acid to produce blue colored intermediate compound nitroso nitroalkanes. But further it does not dissolve in alkali \[NaOH\] , because of the absence of \[\alpha - H\], (alpha hydrogen) .

Coming to \[2 - \]methyl-\[2 - \]-nitropropane, it does not react even with nitrous acid to produce blue colored intermediate compound nitroso nitroalkanes because of the absence of\[\alpha - H\], (alpha hydrogen).
So, here we are clear about the way to distinguish between \[1 - \] nitropropane, \[2 - \]Nitropropane and \[2 - \]methyl-\[2 - \]-nitropropane.
Note:
\[1 - \] nitropropane is used as starting material for the formation of other compounds especially in paint industries. \[2 - \] Nitro propane is used as a solvent or additive in inks, varnishes, polymers. It is used as a solvent and also used in pharmaceutical companies for the formation of compounds like chlorphentermine , teclozan , phentermine . Basically they have a great use in chemical industries also especially in paint based industries .
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




