
Aqueous sulphuric acid reacts with $ 2 $ -methyl- $ 1 $ -butene to give predominantly
(A) Isobutyl hydrogen sulphate
(B) $ 2 $ -methyl- $ 2 $ butanol
(C) $ 2 $ -methyl- $ 1 $ butanol
(D) Secondary butyl hydrogen sulphate
Answer
502.5k+ views
Hint: Alkenes are unsaturated compounds consisting of a double bond between the carbon and carbon atoms. Aqueous sulphuric acid can be used as a dehydrating agent. Thus, alkenes on treatment with sulphuric acid in presence of water undergo Markovnikov rule to form hydroxyl compounds.
Complete answer:
Alcohols are the compounds that consist of hydroxyl groups. Alkenes are unsaturated compounds consisting of a double bond between the carbon and carbon atoms.
Alkenes in presence of aqueous sulphuric acid forms alcohols. Alcohols are also known as hydroxyl compounds. Aqueous sulphuric acid can be used as a hydrating agent.
In this reaction, the water molecule adds to the alkenes, according to the Markovnikov rule. It states that the negative part of the water molecule which is hydroxide ion adds to the carbon with less number of hydrogen atoms. Whereas the positive part of the water molecule which is a proton, adds to the carbon with more hydrogen atoms.
The addition of aqueous sulphuric acid reacts to $ 2 $ -methyl- $ 1 $ -butene will be as follows:
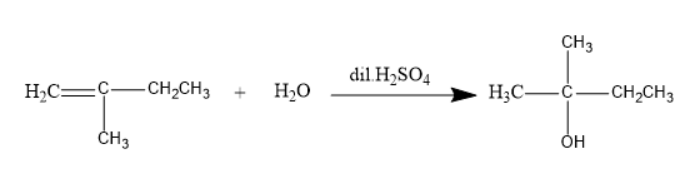
The product formed is $ 2 $ -methyl- $ 2 $ butanol.
Option B is the correct one.
Note:
Addition of alkenes takes place in two ways. One way is the Markovnikov rule and another way is anti- markovnikov rule. Anti- markovnicov rule takes place in presence of peroxides like hydrogen peroxide. In the given reaction there is no peroxide and it takes place according to the Markovnikov rule.
Complete answer:
Alcohols are the compounds that consist of hydroxyl groups. Alkenes are unsaturated compounds consisting of a double bond between the carbon and carbon atoms.
Alkenes in presence of aqueous sulphuric acid forms alcohols. Alcohols are also known as hydroxyl compounds. Aqueous sulphuric acid can be used as a hydrating agent.
In this reaction, the water molecule adds to the alkenes, according to the Markovnikov rule. It states that the negative part of the water molecule which is hydroxide ion adds to the carbon with less number of hydrogen atoms. Whereas the positive part of the water molecule which is a proton, adds to the carbon with more hydrogen atoms.
The addition of aqueous sulphuric acid reacts to $ 2 $ -methyl- $ 1 $ -butene will be as follows:

The product formed is $ 2 $ -methyl- $ 2 $ butanol.
Option B is the correct one.
Note:
Addition of alkenes takes place in two ways. One way is the Markovnikov rule and another way is anti- markovnikov rule. Anti- markovnicov rule takes place in presence of peroxides like hydrogen peroxide. In the given reaction there is no peroxide and it takes place according to the Markovnikov rule.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

State and prove Bernoullis theorem class 11 physics CBSE

Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE

Which out of the following hydrocarbons undergo addition class 11 chemistry CBSE




