
When any substance (except water) is heated, its density:
$
{\text{A}}{\text{. increases}} \\
{\text{B}}{\text{. decreases}} \\
{\text{C}}{\text{. remains same}} \\
{\text{D}}{\text{. none of these}} \\
$
Answer
598.2k+ views
Hint: Density is the ratio of mass and volume of the substance. So the change caused by temperature on mass and volume should be checked, to now the effect on density.
Complete step-by-step answer:
Mass is a property that is fixed. It does not change unless relativity applies. So, on increasing temperature, the mass remains unaffected.
In general, all substances (except water) expand on heating. As on heating, the internal energy of a substance increases. There are vibrations in molecules and therefore, the molecular distances increase. As the distance between molecules is increasing, the substance occupies more space. Hence, as the temperature is increased so their volume increases.
So, we can generalize that:
With an increase in temperature:
Mass remains unaffected
Volume increases
As, $density = \dfrac{{mass}}{{volume}}$
In the diffraction, the numerator remains constant but the denominator is increasing. As a whole, the diffraction (=density) is decreasing.
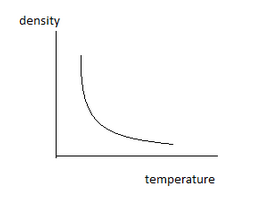
So density decreases.
Correct option is (B).
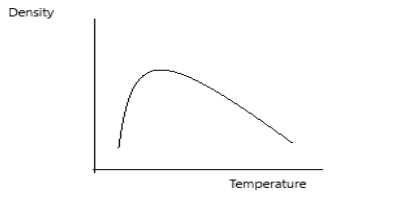
Note: In case of water, the expansion of volume follows a different trend. So the variation in density with temperature will be different.
The variation in density for water can be shown as:
Complete step-by-step answer:
Mass is a property that is fixed. It does not change unless relativity applies. So, on increasing temperature, the mass remains unaffected.
In general, all substances (except water) expand on heating. As on heating, the internal energy of a substance increases. There are vibrations in molecules and therefore, the molecular distances increase. As the distance between molecules is increasing, the substance occupies more space. Hence, as the temperature is increased so their volume increases.
So, we can generalize that:
With an increase in temperature:
Mass remains unaffected
Volume increases
As, $density = \dfrac{{mass}}{{volume}}$
In the diffraction, the numerator remains constant but the denominator is increasing. As a whole, the diffraction (=density) is decreasing.

So density decreases.
Correct option is (B).
Note: In case of water, the expansion of volume follows a different trend. So the variation in density with temperature will be different.
The variation in density for water can be shown as:

Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

State and prove converse of BPT Basic Proportionality class 10 maths CBSE




