
Aniline reacts with phosgene and excess of $ K\;OH $ to form :
(A)
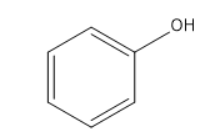
(B)
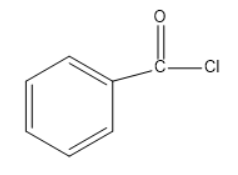
(C)
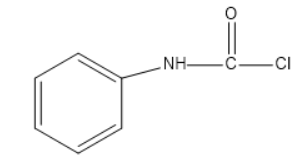
(D)
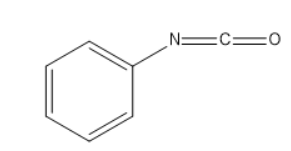




Answer
517.8k+ views
Hint :Try to solve this question by drawing the structure of phosgene and aniline in the presence of $ K\;OH $ taking into consideration the dipole moment, effect of presence of lone pair, polarity of bonds etc. This way you’ll be able to decipher the mechanism of the above reaction.
Complete Step By Step Answer:
Before the mechanism of the above reaction let us first familiarise ourselves with the structure of the compounds given in the question.
Aniline-
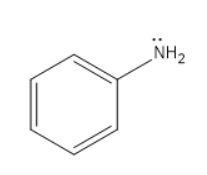
Aniline is the organic compound which consists of a phenyl group attached to an amino group. The amino group contains a lone pair which will participate in the above said chemical reaction.
Phosgene-
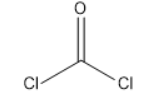
Mechanism:
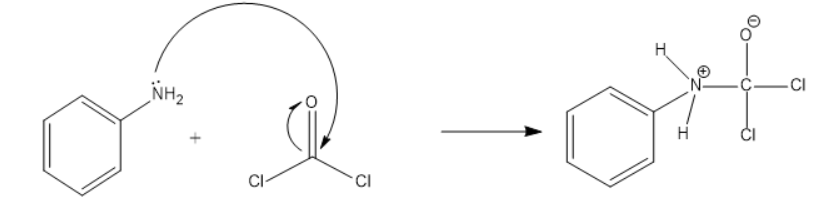
The lone pair on amine attacks the C of the phosgene and due to oxygen’s electronegativity the polarity moves towards the oxygen. The above product is formed. This negative charge on the oxygen will back off and will form a double bond which results in the release of Cl. This is shown below:
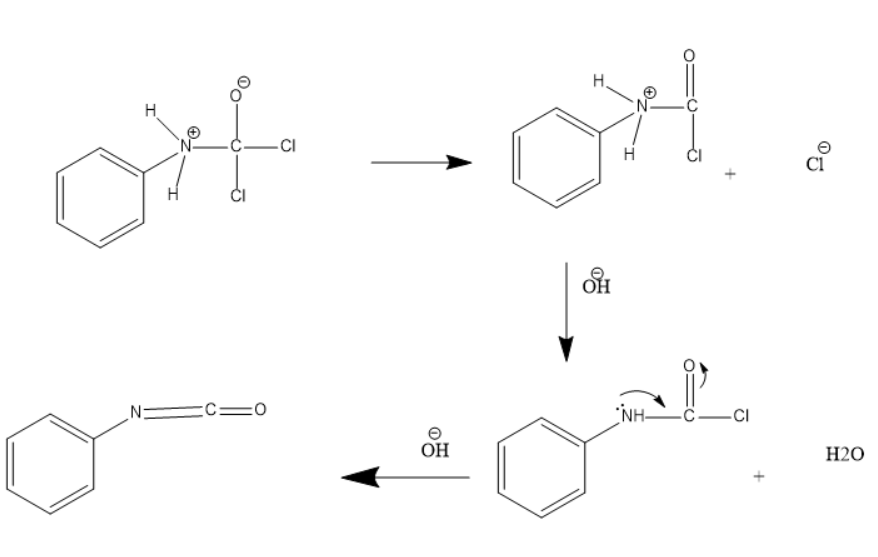
Then the $ ClO{H^{-}} $ from potassium hydroxide attacks the above intermediate product which further results in release of water. On further reaction of the $ O{H^{-} $ ion which results in the formation of phenyl isocyanate.
The intermediate formed in the above reactions are unstable and therefore undergo rearrangement to achieve a stable structure.
Therefore our answer is D.
Note :
Option a and b does occur in the above reaction but only as the intermediate not the final product. Also $ K\;OH $ can be written as $ {K^ + }O{H^{^ - }} $ . The $ O{H^{^ - }} $ in the above mechanism comes when the polarity of the potassium hydroxide is depicted individually.
Complete Step By Step Answer:
Before the mechanism of the above reaction let us first familiarise ourselves with the structure of the compounds given in the question.
Aniline-

Aniline is the organic compound which consists of a phenyl group attached to an amino group. The amino group contains a lone pair which will participate in the above said chemical reaction.
Phosgene-

Mechanism:

The lone pair on amine attacks the C of the phosgene and due to oxygen’s electronegativity the polarity moves towards the oxygen. The above product is formed. This negative charge on the oxygen will back off and will form a double bond which results in the release of Cl. This is shown below:

Then the $ ClO{H^{-}} $ from potassium hydroxide attacks the above intermediate product which further results in release of water. On further reaction of the $ O{H^{-} $ ion which results in the formation of phenyl isocyanate.
The intermediate formed in the above reactions are unstable and therefore undergo rearrangement to achieve a stable structure.
Therefore our answer is D.
Note :
Option a and b does occur in the above reaction but only as the intermediate not the final product. Also $ K\;OH $ can be written as $ {K^ + }O{H^{^ - }} $ . The $ O{H^{^ - }} $ in the above mechanism comes when the polarity of the potassium hydroxide is depicted individually.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




