
Aniline is soluble in which of the following organic reagents?
a.) Benzene
b.) Ether
c.) Alcohol
d.) All of the above
Answer
581.4k+ views
Hint: Try to analyze the physical properties of the aniline. Solubility is also a physical property which says how ease a compound can dissolve or mix in another compound. Also discuss the nature of the groups that are attached to the benzene ring in the aniline molecule.
Complete Solution :
The molecular formula of the aniline is ${{C}_{6}}{{H}_{5}}-N{{H}_{2}}$ which is having a typical structure as shown below
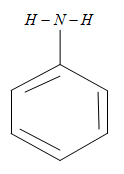
The polarity of this molecule is very low and also the intermolecular hydrogen bonding in itself is not that much strong so that it can form a hydrogen bonding with itself which makes it not to dissolve in any organic molecule. These properties make the aniline to solve in the organic molecules and it can also form the hydrogen bonding with the molecules of the other molecules other than the aniline. And hence aniline can dissolve in organic reagents like benzene, ether, alcohol. That means aniline can soluble in all the organic reagents given above.
So, the correct answer is “Option D”.
Note: In non polar solvents like \[C{{S}_{2}}\] the aniline will exhibit high resonance and due to this it becomes ready to undergo reduction. Due to this effect the ortho and the Para positions are substituted by reducing the $N{{H}_{2}}$ group that is attached in the aniline and the process is called as the mono-substitution.
Complete Solution :
The molecular formula of the aniline is ${{C}_{6}}{{H}_{5}}-N{{H}_{2}}$ which is having a typical structure as shown below

The polarity of this molecule is very low and also the intermolecular hydrogen bonding in itself is not that much strong so that it can form a hydrogen bonding with itself which makes it not to dissolve in any organic molecule. These properties make the aniline to solve in the organic molecules and it can also form the hydrogen bonding with the molecules of the other molecules other than the aniline. And hence aniline can dissolve in organic reagents like benzene, ether, alcohol. That means aniline can soluble in all the organic reagents given above.
So, the correct answer is “Option D”.
Note: In non polar solvents like \[C{{S}_{2}}\] the aniline will exhibit high resonance and due to this it becomes ready to undergo reduction. Due to this effect the ortho and the Para positions are substituted by reducing the $N{{H}_{2}}$ group that is attached in the aniline and the process is called as the mono-substitution.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




