
An organic compound (A) of molecular formula \[{{C}_{3}}{{H}_{8}}O\] gives turbidity within 5-10 min on reaction with anhydrous $ZnC{{l}_{2}}/HCl$. Compound (A) on treatment with sodium hypochlorite gives a carbonyl compound (B) which on further chlorination gives compound (C) of molecular formula ${{C}_{3}}{{H}_{3}}OC{{l}_{3}}$. Identify (A), (B), and (C). Explain the reactions.
Answer
564.6k+ views
Hint: Isopropyl alcohol is a colourless and flammable liquid. When this compound reacts with sodium hypochlorite, it gives ketone group which on further chlorination shows ester group formation.
Complete answer:
- In the question, we have the molecular formula of the compound (A), i.e. \[{{C}_{3}}{{H}_{8}}O\]. So, the molecular structure would be
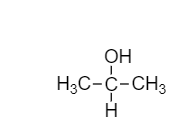
- This is our compound (A) Methoxy ethane or Isopropyl alcohol.
-As we know, this compound gives turbidity within 5-10 min on reaction with anhydrous $ZnC{{l}_{2}}/HCl$. On treatment with Sodium hypochlorite $NaOCl$, we get compound (B)
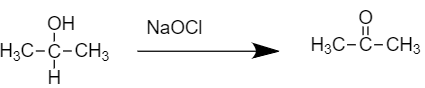
- The resulting product is our compound (B) Acetone.
- Compound (B), on further chlorination gives compound (C) with the molecular formula${{C}_{3}}{{H}_{3}}OC{{l}_{3}}$. So, the reaction would be as shown below
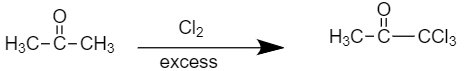
The final product is our compound (C) Methylchloroglyoxylate.
Additional information:
Sodium hypochlorite, on ingestion, can show minor damage to the esophagus, causing first-degree burns with hyperemia and edema of the mucosa. $ZnC{{l}_{2}}$ is a water soluble, highly hygroscopic compound. It finds wide variety under the textile industry and chemical synthesis.
Note:
Sodium hypochlorite is commonly known as bleach. It is frequently used as a disinfecting agent and very effective for the disinfection of viruses, bacteria, fungi, and mycobacterium. However, sodium hypochlorite is NOT effective in the disinfection of bacterial spores and prions.
Complete answer:
- In the question, we have the molecular formula of the compound (A), i.e. \[{{C}_{3}}{{H}_{8}}O\]. So, the molecular structure would be

- This is our compound (A) Methoxy ethane or Isopropyl alcohol.
-As we know, this compound gives turbidity within 5-10 min on reaction with anhydrous $ZnC{{l}_{2}}/HCl$. On treatment with Sodium hypochlorite $NaOCl$, we get compound (B)

- The resulting product is our compound (B) Acetone.
- Compound (B), on further chlorination gives compound (C) with the molecular formula${{C}_{3}}{{H}_{3}}OC{{l}_{3}}$. So, the reaction would be as shown below

The final product is our compound (C) Methylchloroglyoxylate.
Additional information:
Sodium hypochlorite, on ingestion, can show minor damage to the esophagus, causing first-degree burns with hyperemia and edema of the mucosa. $ZnC{{l}_{2}}$ is a water soluble, highly hygroscopic compound. It finds wide variety under the textile industry and chemical synthesis.
Note:
Sodium hypochlorite is commonly known as bleach. It is frequently used as a disinfecting agent and very effective for the disinfection of viruses, bacteria, fungi, and mycobacterium. However, sodium hypochlorite is NOT effective in the disinfection of bacterial spores and prions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




