
An isomer of ethanol is:
A. Methanol
B. Dimethyl ether
C. Acetone
D. Diethyl ether
Answer
583.2k+ views
Hint: Shape of a molecule in space contributes to its properties. Its shape depends on how its atoms are arranged in the space. Keeping the molecular formula, the same, when the atoms of the molecule are arranged differently, they are called isomers.
Complete step by step answer: Ethanol has molecular formula ${C_2}{H_5}OH{\text{ or }}{C_2}{H_6}O$, and it has the following structure:
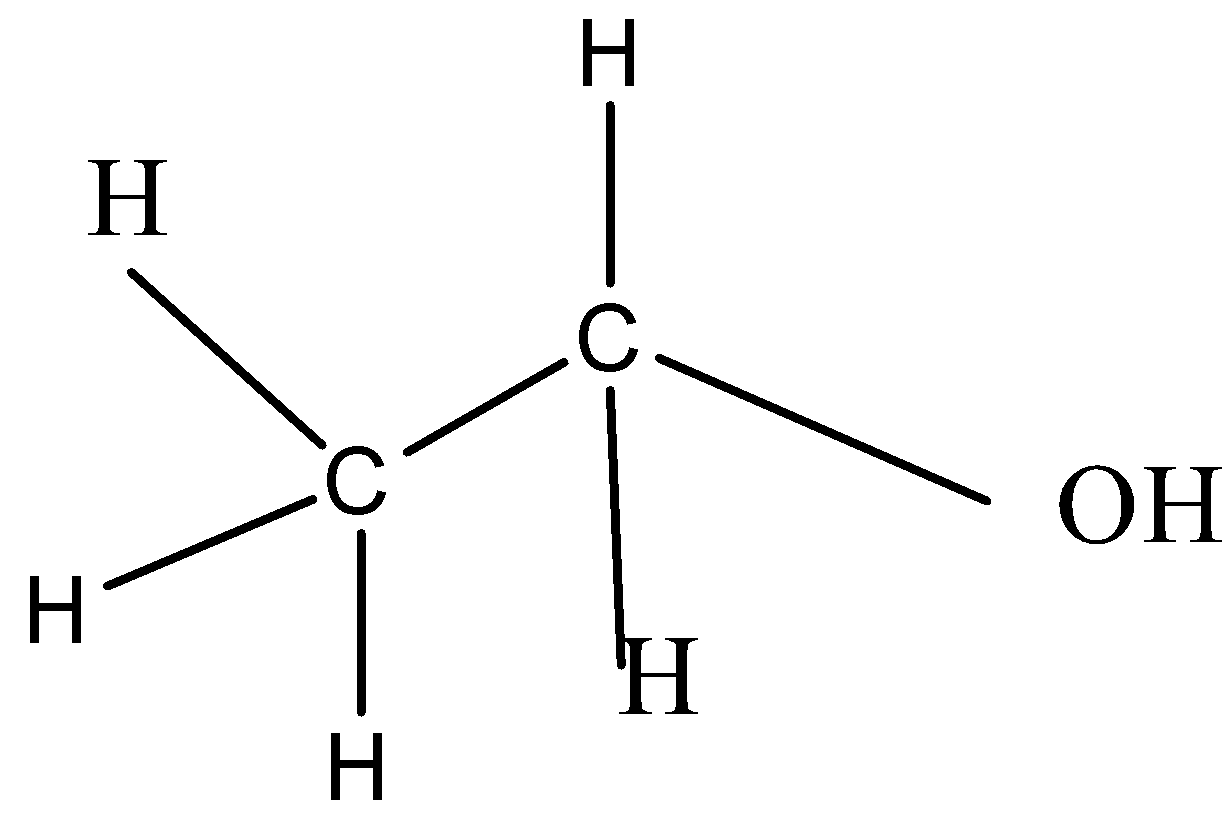
The isomer of ethanol should have the same molecular formula but different arrangement of atoms or different structure. This difference can be from the presence of different functional groups attached to the molecules. Molecules having the same molecular formula but different functional groups are called functional group isomers.
-Methanol- It has the molecular formula $C{H_3}OH{\text{ or }}C{H_4}O$. So, it does have the same molecular formula as ethanol. Hence, option (a) is incorrect.
-Dimethyl Ether- It has a molecular formula $C{H_3} - O - C{H_3}{\text{ or }}{C_2}{H_6}O$. So, it has the same molecular formula as ethanol, C2H6O. Now, we have to check if it also has a different arrangement of atoms than ethanol or not. The structure of dimethyl ether is:

Thus, dimethyl ether has the same molecular formula as ethanol but different structure due to different functional groups. Hence, option (b) is the correct match.
3) Acetone- It has the molecular formula $C{H_3}COC{H_3}{\text{ or }}{C_3}{H_6}O$. So, it does not have the same molecular formula as ethanol and hence, not an isomer.
4) Diethyl Ether- It is a molecular formula ${C_2}{H_5} - O - {C_2}{H_5}{\text{ or }}{C_4}{H_{10}}O$, which does not match the formula of ethanol. Hence, it is not an isomer of ethanol.
Hence, option (b) dimethyl ether is correct.
Note: For identifying structural or constitutional isomers, look for their bonding pattern. The atoms of the compound will be the same but, they are connected differently to make different functional groups. Example: n-butane and isobutene.
Complete step by step answer: Ethanol has molecular formula ${C_2}{H_5}OH{\text{ or }}{C_2}{H_6}O$, and it has the following structure:

The isomer of ethanol should have the same molecular formula but different arrangement of atoms or different structure. This difference can be from the presence of different functional groups attached to the molecules. Molecules having the same molecular formula but different functional groups are called functional group isomers.
-Methanol- It has the molecular formula $C{H_3}OH{\text{ or }}C{H_4}O$. So, it does have the same molecular formula as ethanol. Hence, option (a) is incorrect.
-Dimethyl Ether- It has a molecular formula $C{H_3} - O - C{H_3}{\text{ or }}{C_2}{H_6}O$. So, it has the same molecular formula as ethanol, C2H6O. Now, we have to check if it also has a different arrangement of atoms than ethanol or not. The structure of dimethyl ether is:

Thus, dimethyl ether has the same molecular formula as ethanol but different structure due to different functional groups. Hence, option (b) is the correct match.
3) Acetone- It has the molecular formula $C{H_3}COC{H_3}{\text{ or }}{C_3}{H_6}O$. So, it does not have the same molecular formula as ethanol and hence, not an isomer.
4) Diethyl Ether- It is a molecular formula ${C_2}{H_5} - O - {C_2}{H_5}{\text{ or }}{C_4}{H_{10}}O$, which does not match the formula of ethanol. Hence, it is not an isomer of ethanol.
Hence, option (b) dimethyl ether is correct.
Note: For identifying structural or constitutional isomers, look for their bonding pattern. The atoms of the compound will be the same but, they are connected differently to make different functional groups. Example: n-butane and isobutene.

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




