
An ethereal solution of alkyl halide is heated with sodium metal. The reaction is known as:
A.Franklands reaction
B. Sandmeyer’s reaction
C. Wurtz-Fittig reaction
D. Wurtz reaction
Answer
572.1k+ views
Hint: The reactions which are undergone by alkyl halides need to be learnt for answering this question. Alkyl halides have differences in charges, which makes them polar and prone to reactions involving nuclear substitution.
Complete Solution :
In order to answer our question, we need to learn about the properties of alkyl halides. In an alkyl halide, a halogen atom is attached to the carbon chain. A difference in charges is created and a partial negative charge appears on the halogen atom which makes it prone to nucleophilic reactions. The C-X bond of haloalkanes is polar with partial positive charge on carbon and partial negative charge on halogen. Any nucleophile stronger than halide ion can attack at the C-atom due to positive charge causing nucleophilic substitution. Halide ions have weak bases and are good leaving groups, thus haloalkanes undergo elimination reaction with a strong base. Reactive metals cause cleavage of C-X bond forming organometallic compounds. In nucleophilic substitution reactions, the incoming nucleophile having at least one atom with a lone pair of electrons attacks at the carbon atom bonded to halogen. The halide ion leaves with the bond pair of electrons. Now, during the Wurtz reaction, the partially negatively charged halogen X donate its radical to the carbon chain and forms a bond with sodium forming NaX. Then the positively charged ‘R’ of the R - X occupies the position of X. The mechanism of Wurtz Fittig reaction is shown below:
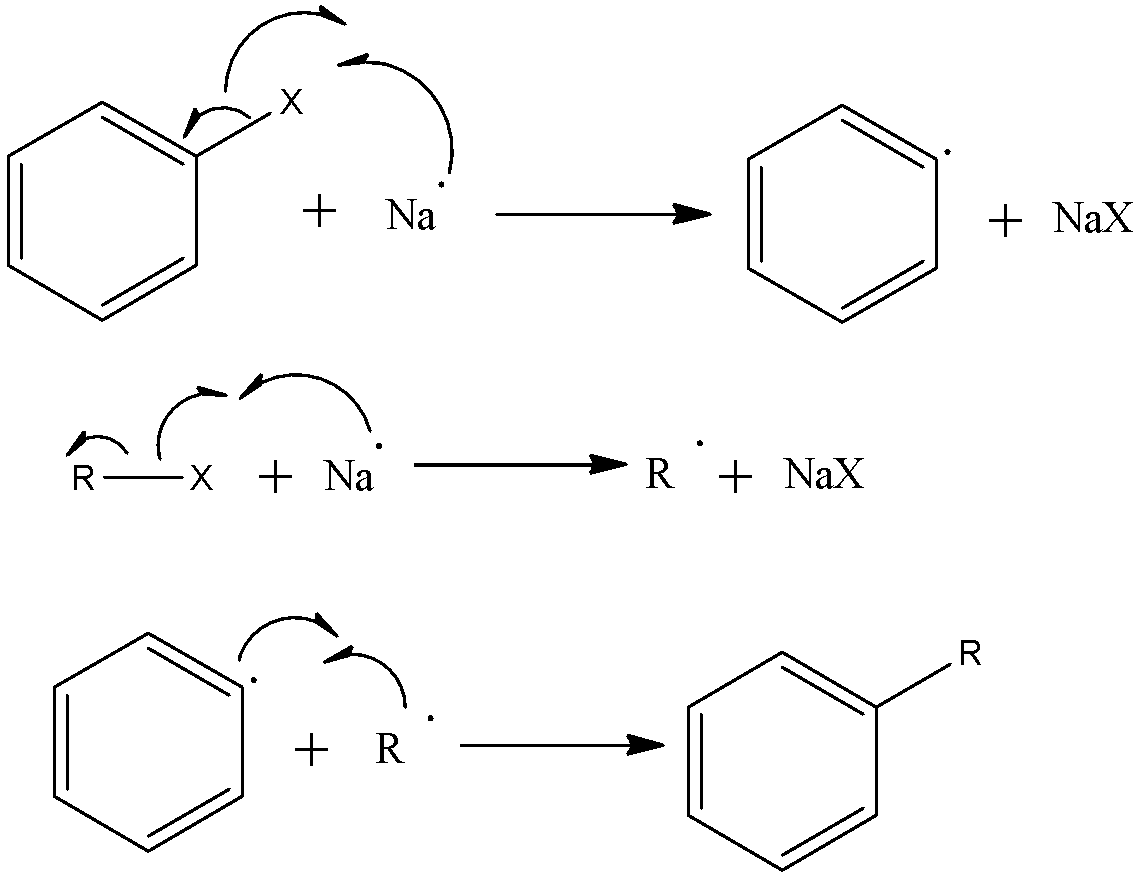
So, when an ethereal solution of only an alkyl halide is heated with sodium metal, then the reaction is called Wurtz reaction So, the correct answer is “Option D”.
Note: The Wurtz Reaction and Wurtz-Fittig reactions are exactly similar and also possess the same mechanism. It is just that in case of Wurtz Fittig reaction, an aryl halide and alkyl halide is taken, whereas in Wurtz reaction, only alkyl halide is taken.
Complete Solution :
In order to answer our question, we need to learn about the properties of alkyl halides. In an alkyl halide, a halogen atom is attached to the carbon chain. A difference in charges is created and a partial negative charge appears on the halogen atom which makes it prone to nucleophilic reactions. The C-X bond of haloalkanes is polar with partial positive charge on carbon and partial negative charge on halogen. Any nucleophile stronger than halide ion can attack at the C-atom due to positive charge causing nucleophilic substitution. Halide ions have weak bases and are good leaving groups, thus haloalkanes undergo elimination reaction with a strong base. Reactive metals cause cleavage of C-X bond forming organometallic compounds. In nucleophilic substitution reactions, the incoming nucleophile having at least one atom with a lone pair of electrons attacks at the carbon atom bonded to halogen. The halide ion leaves with the bond pair of electrons. Now, during the Wurtz reaction, the partially negatively charged halogen X donate its radical to the carbon chain and forms a bond with sodium forming NaX. Then the positively charged ‘R’ of the R - X occupies the position of X. The mechanism of Wurtz Fittig reaction is shown below:

So, when an ethereal solution of only an alkyl halide is heated with sodium metal, then the reaction is called Wurtz reaction So, the correct answer is “Option D”.
Note: The Wurtz Reaction and Wurtz-Fittig reactions are exactly similar and also possess the same mechanism. It is just that in case of Wurtz Fittig reaction, an aryl halide and alkyl halide is taken, whereas in Wurtz reaction, only alkyl halide is taken.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




