
An element A is tetravalent and another element B is divalent. The formula of the compound formed by the combination of these elements is:
A. \[{A_2}B\]
B. \[AB\]
C. \[A{B_2}\]
D. \[{A_2}{B_3}\]
Answer
582.9k+ views
Hint: The molecular formula of a compound represents the atoms and the number of atoms present in the compound. The number of the atoms in the compound are written in the form of suffix. A structural formula of a compound represents the structure of a particular compound.
Complete step by step answer:
We are given that an element A is tetravalent, hence its oxidation number can be \[{{\text{A}}^{{\text{ + 4}}}}\]
Also, it is also given that element B is divalent hence its oxidation number can be \[{{\text{B}}^{{\text{2}} - }}\].
Therefore, we give the formula of the compound by cross method as,
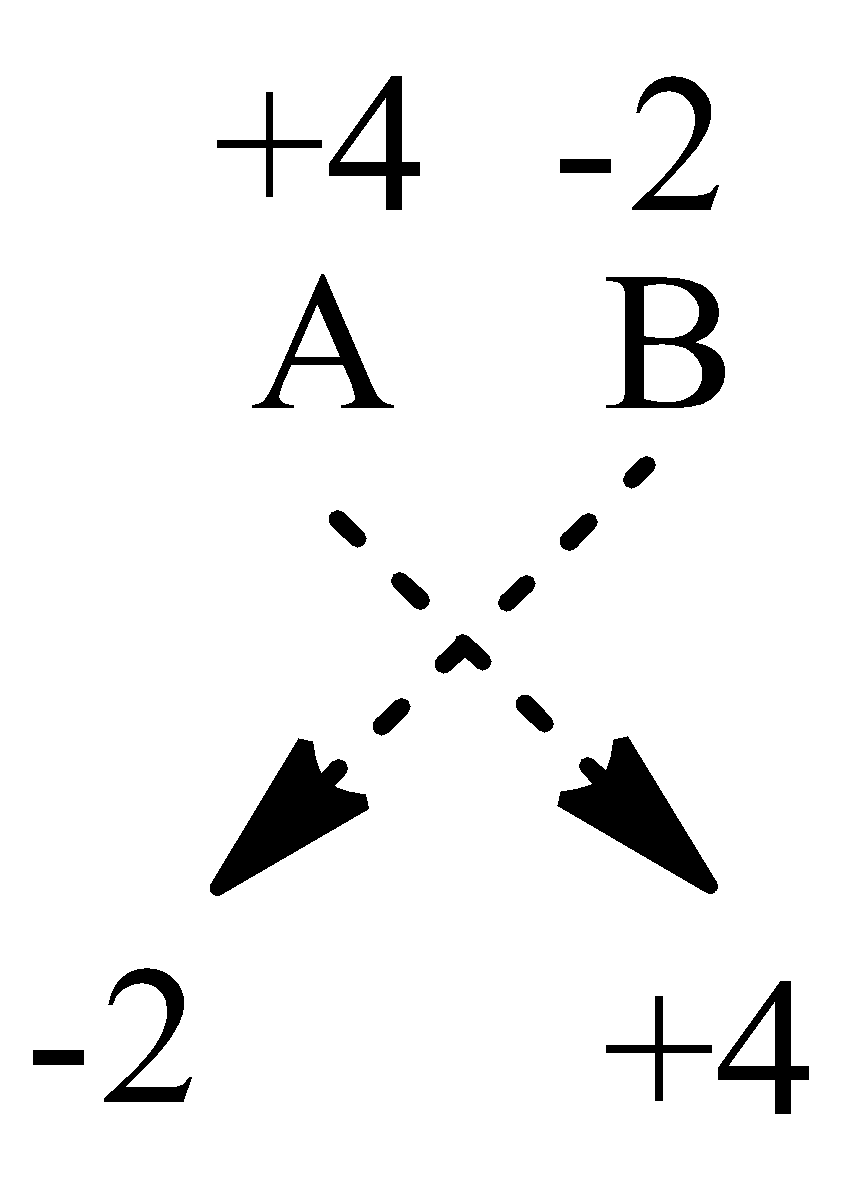
So, the formula of the compound will be \[{{\text{A}}_{\text{2}}}{{\text{B}}_{\text{4}}}\]or \[{\text{A}}{{\text{B}}_2}\].
So, the correct answer is Option C .
Note:
In chemical compounds, the elements are present in a fixed ratio by mass. This was given by Joseph Proust. This is the “law of constant proportions''. This “law of constant proportions” is also termed as the law of definite proportions or as the Proust’s law. For example, pure water contains oxygen and hydrogen in a fixed ratio of 1:8. Water contains 0.88 grams of oxygen and 0.11 grams of hydrogen
Students may get confused between formula units and molecular formulas. By formula unit we represent the ratio of the ions which is present in a compound. The molecular formula of a compound represents the atoms and the number of atoms present in the compound. The number of the atoms in the compound are written in the form of suffix.
Complete step by step answer:
We are given that an element A is tetravalent, hence its oxidation number can be \[{{\text{A}}^{{\text{ + 4}}}}\]
Also, it is also given that element B is divalent hence its oxidation number can be \[{{\text{B}}^{{\text{2}} - }}\].
Therefore, we give the formula of the compound by cross method as,

So, the formula of the compound will be \[{{\text{A}}_{\text{2}}}{{\text{B}}_{\text{4}}}\]or \[{\text{A}}{{\text{B}}_2}\].
So, the correct answer is Option C .
Note:
In chemical compounds, the elements are present in a fixed ratio by mass. This was given by Joseph Proust. This is the “law of constant proportions''. This “law of constant proportions” is also termed as the law of definite proportions or as the Proust’s law. For example, pure water contains oxygen and hydrogen in a fixed ratio of 1:8. Water contains 0.88 grams of oxygen and 0.11 grams of hydrogen
Students may get confused between formula units and molecular formulas. By formula unit we represent the ratio of the ions which is present in a compound. The molecular formula of a compound represents the atoms and the number of atoms present in the compound. The number of the atoms in the compound are written in the form of suffix.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




