
An alcohol of molecular formula \[{C_5}{H_{14}}OH\] on dehydration gives an alkene, which on oxidation yields a mixture of ketone and an acid. The alcohol is:
A. \[C{H_3}C{H_2}CH(OH)C{H_2}C{H_3}\]
B. \[C{H_2} - \mathop {\mathop C\limits_| }\limits_{OH} HC{H_2}C{H_2}C{H_3}\]
C. \[{(C{H_3})_2}CHCH(OH)C{H_3}\]
D. \[{(C{H_3})_3}CC{H_2}OH\]
Answer
564.6k+ views
Hint: The alcohol \[{C_5}{H_{14}}OH\] undergoes dehydration using sulphuric acid to produce alkene with molecular formula \[{(C{H_3})_2}CCHC{H_3}\] which on oxidation with potassium permanganate gives a carboxylic acid and a ketone.
Complete step by step answer:Given,
The molecular formula of alcohol is \[{C_5}{H_{14}}OH\].
It is given that the alcohol on dehydration gives alkene which further on oxidation gives mixture of ketone and an acid as the main product.
Dehydration is the reaction used for the conversion reactant into product by removing water and using a dehydrating reagent. This reaction is the reverse of the hydration reaction where a water molecule is added to the reactant to form the product.
In oxidation reaction, the carbon atoms gain electrons from electronegative atoms mostly oxygen.
The reaction of alcohol with sulphuric acid and further oxidizing with potassium permanganate is shown below
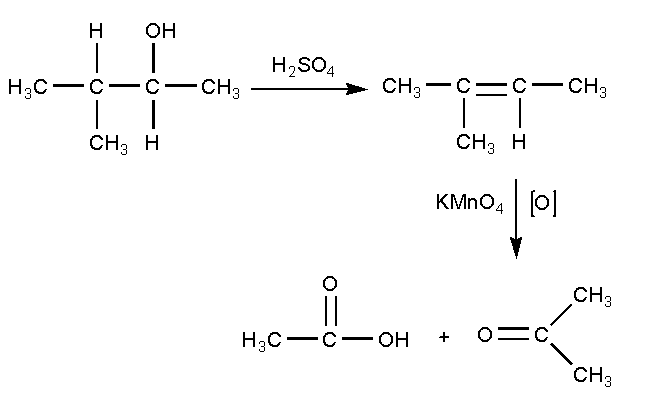
In this reaction, first 3-methylbutan-2-ol reacts with sulphuric acid \[{H_2}S{O_4}\] where the lone pairs present on the oxygen of hydroxyl group abstract the hydrogen of sulphuric acid and bond will shift to oxygen forming an anion. Further the water molecule will be removed and the bond will shift to form an alkene product. The product formed is 2-methyl-but-2-ene. The compound 2-methyl-but-2-ene is oxidized using oxidizing agent potassium permanganate to give a carboxylic acid and a ketone. The carboxylic acid product is ethanoic acid and the ketone product is propan-2-one.
Therefore, the alcohol with molecular weight \[{C_5}{H_{14}}OH\] is \[{(C{H_3})_2}CHCH(OH)C{H_3}\].
Therefore, the correct option is C.
Note:
Apart from sulphuric acid, the other dehydrating agent is concentrated phosphoric acid, hot aluminium oxide. The other oxidizing agent is hydrogen peroxide, ozone, nitric acid.
Complete step by step answer:Given,
The molecular formula of alcohol is \[{C_5}{H_{14}}OH\].
It is given that the alcohol on dehydration gives alkene which further on oxidation gives mixture of ketone and an acid as the main product.
Dehydration is the reaction used for the conversion reactant into product by removing water and using a dehydrating reagent. This reaction is the reverse of the hydration reaction where a water molecule is added to the reactant to form the product.
In oxidation reaction, the carbon atoms gain electrons from electronegative atoms mostly oxygen.
The reaction of alcohol with sulphuric acid and further oxidizing with potassium permanganate is shown below

In this reaction, first 3-methylbutan-2-ol reacts with sulphuric acid \[{H_2}S{O_4}\] where the lone pairs present on the oxygen of hydroxyl group abstract the hydrogen of sulphuric acid and bond will shift to oxygen forming an anion. Further the water molecule will be removed and the bond will shift to form an alkene product. The product formed is 2-methyl-but-2-ene. The compound 2-methyl-but-2-ene is oxidized using oxidizing agent potassium permanganate to give a carboxylic acid and a ketone. The carboxylic acid product is ethanoic acid and the ketone product is propan-2-one.
Therefore, the alcohol with molecular weight \[{C_5}{H_{14}}OH\] is \[{(C{H_3})_2}CHCH(OH)C{H_3}\].
Therefore, the correct option is C.
Note:
Apart from sulphuric acid, the other dehydrating agent is concentrated phosphoric acid, hot aluminium oxide. The other oxidizing agent is hydrogen peroxide, ozone, nitric acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




