
What is the amount of energy needed to start a chemical reaction?
Answer
528k+ views
Hint: A chemical reaction takes place only when the molecules are moving with high kinetic energy and has proper orientation for collision with other molecules. The movement of molecules and breaking of bonds require some initial amount of energy to activate or start the reaction.
Complete answer:
A chemical reaction is a process in which atoms of a few or more molecules rearrange and combine to create a new species. The combining molecules are called reactants and the produced substances are called the products.
During the chemical change from a reactant to a product, bonds present between individual reactant molecules are being broken and new bond formation takes place. The energy is needed to overcome the intermolecular attractions and break the reactant’s bond. Also, the energy is required by molecules to gain high kinetic energy. Because, when the energy is high, the atoms’ movement will be faster and random and this will lead to a greater number of effective collisions between molecules.
This minimum amount of energy required to initiate a chemical reaction is known as activation energy. All chemical reactions require energy to initiate. Even the exothermic reactions require some amount of energy to get started and once it initiates, it releases energy to activate the further reaction. The peak of the graph shown below indicates the activation energy required by the reaction to initiate and transform reactants into products.
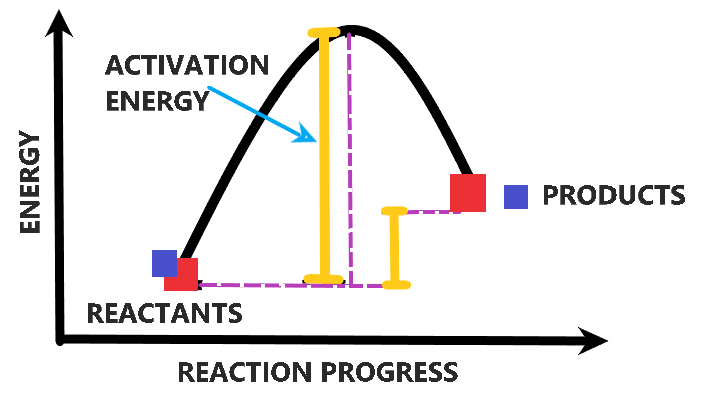
Thus, the amount of energy needed to start a chemical reaction is called activation energy.
Note:
Any chemical reaction will only occur when molecules collide with sufficient energy, that is activation energy and with proper orientation so that chemical bonds can break. A collision that satisfies these two criteria and results in a successful reaction is known as an effective collision.
Complete answer:
A chemical reaction is a process in which atoms of a few or more molecules rearrange and combine to create a new species. The combining molecules are called reactants and the produced substances are called the products.
During the chemical change from a reactant to a product, bonds present between individual reactant molecules are being broken and new bond formation takes place. The energy is needed to overcome the intermolecular attractions and break the reactant’s bond. Also, the energy is required by molecules to gain high kinetic energy. Because, when the energy is high, the atoms’ movement will be faster and random and this will lead to a greater number of effective collisions between molecules.
This minimum amount of energy required to initiate a chemical reaction is known as activation energy. All chemical reactions require energy to initiate. Even the exothermic reactions require some amount of energy to get started and once it initiates, it releases energy to activate the further reaction. The peak of the graph shown below indicates the activation energy required by the reaction to initiate and transform reactants into products.

Thus, the amount of energy needed to start a chemical reaction is called activation energy.
Note:
Any chemical reaction will only occur when molecules collide with sufficient energy, that is activation energy and with proper orientation so that chemical bonds can break. A collision that satisfies these two criteria and results in a successful reaction is known as an effective collision.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




