
Amongst the given options, the compound(s) in which all the atoms are in one plane in all the possible conformations (if any), is/are:
(A)
(B)
(C)
(D)
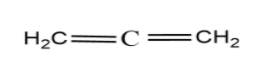



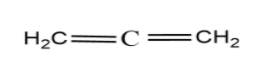
Answer
577.2k+ views
Hint: Conformations of any given compound are all possible structures the compound can be arranged in different planes to get stability. To know the planarity of a compound one has to consider the rotation of single bonds between carbon atoms.
Complete step by step solution:
Let us find out the planarity in the structure of all given options.
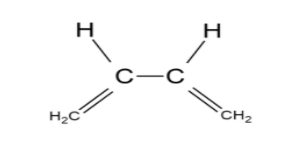
The compound (A) is named 1,3 butadiene. There is a single bond between C-2 carbon atom and C-3 carbon atom, so rotation about the bond is possible forming different conformations. The given conformation of 1,3-butadiene is the cis isomer of butadiene where similar groups are placed adjacent to each other. But the s cis-butadiene is non-planar with dihedral angle of ${38^ \circ }$.
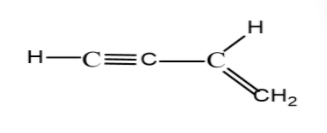
The compound (B) is 1-butene-3-yne. The structural arrangement of the atoms as shown itself is planar for but-3-yne. Because upon rotation of the bond, the alkyne ${\text{C}} \equiv {\text{C}}$ group rotates than the alkene group ${\text{C = C}}$, as the alkyne is attached to oxygen atom at its end, the oxygen still remains in the plane. So, the molecule is planar, if rotated, or if not rotated.
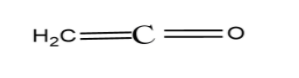
The compound (C) is known as ketene. Ketene is absolutely a planar molecule because the hybridization of the central carbon atom is sp hybridization and the molecule gets linear symmetry when it is sp hybridized.
The compound (D) is called allene and it has a non-planar structure. The hydrogen atoms attached to two terminal carbons do not lie in the same plane; instead they lie perpendicular to each other to minimize the repulsions.
So the answers for the above question are option (B) and option (C).
Note: There is a C2-C3 single bond in 1-butene-3-yne along which rotation is possible but this rotation does not change its stereochemistry of the molecule. Also all the atoms of ketene align in the same plane as it is a straight molecule.
Complete step by step solution:
Let us find out the planarity in the structure of all given options.
The compound (A) is named 1,3 butadiene. There is a single bond between C-2 carbon atom and C-3 carbon atom, so rotation about the bond is possible forming different conformations. The given conformation of 1,3-butadiene is the cis isomer of butadiene where similar groups are placed adjacent to each other. But the s cis-butadiene is non-planar with dihedral angle of ${38^ \circ }$.
The compound (B) is 1-butene-3-yne. The structural arrangement of the atoms as shown itself is planar for but-3-yne. Because upon rotation of the bond, the alkyne ${\text{C}} \equiv {\text{C}}$ group rotates than the alkene group ${\text{C = C}}$, as the alkyne is attached to oxygen atom at its end, the oxygen still remains in the plane. So, the molecule is planar, if rotated, or if not rotated.
The compound (C) is known as ketene. Ketene is absolutely a planar molecule because the hybridization of the central carbon atom is sp hybridization and the molecule gets linear symmetry when it is sp hybridized.
The compound (D) is called allene and it has a non-planar structure. The hydrogen atoms attached to two terminal carbons do not lie in the same plane; instead they lie perpendicular to each other to minimize the repulsions.
So the answers for the above question are option (B) and option (C).
Note: There is a C2-C3 single bond in 1-butene-3-yne along which rotation is possible but this rotation does not change its stereochemistry of the molecule. Also all the atoms of ketene align in the same plane as it is a straight molecule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




