
All of the following reactions are non-elementary reactions.
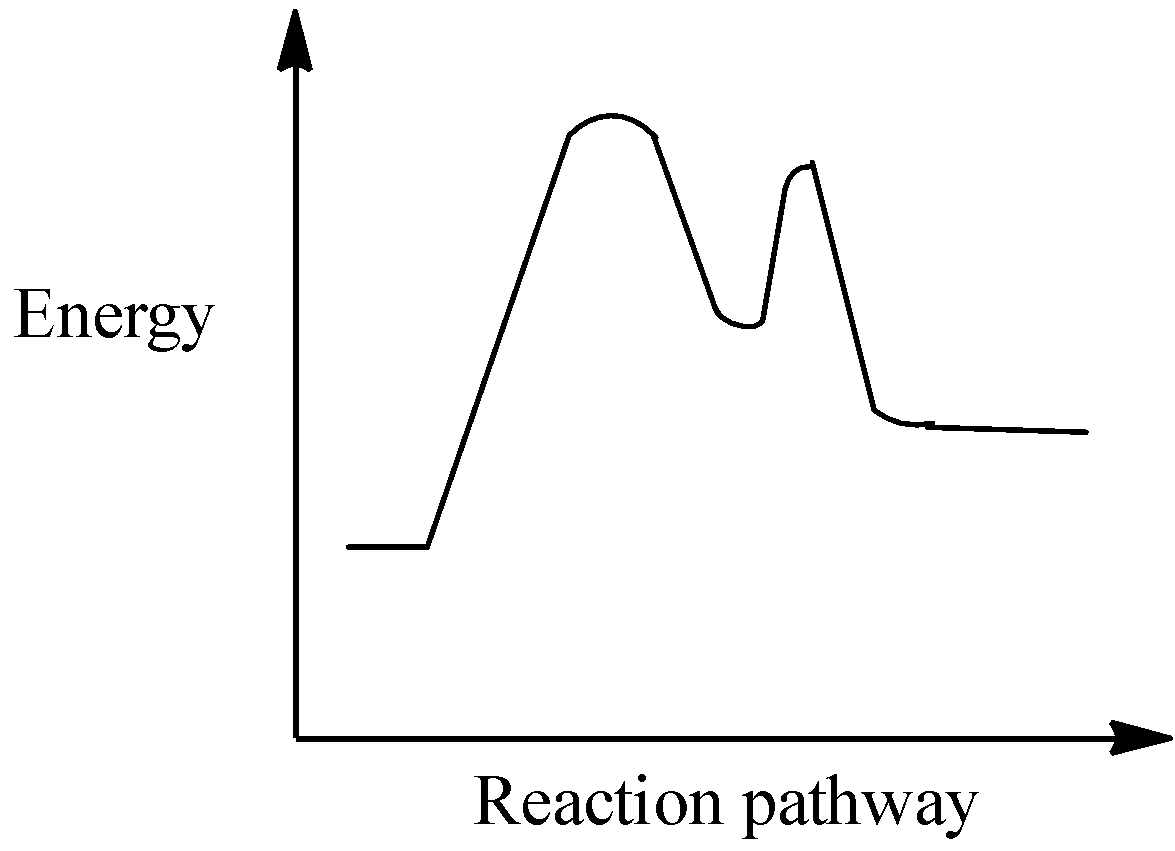
Which of the following is the best fit reaction for the following energy diagram?
A) The reaction between aluminium and copper (II) sulphate solution with a $\Delta H = -12.37 kJ/mol$
B) The reaction that forms hydrogen and nitrogen from ammonia all in the gas state with a $\Delta H = 30.50 kJ/mol$
C) The dissolution of sodium hydroxide in water with a$\Delta H = -44.51kJ/mol$
D) The ionisation of hydrochloric acid to make a solution with a$\Delta H = -74.84kJ/mol$

Answer
565.2k+ views
Hint: The answer to this question is based on the concept of potential energy diagram and therefore depends on the comparison energy of the reactants and product which assigns either positive or negative value to the enthalpy of reaction.
Complete Solution :
The concept of the potential energy diagram is familiar to us from the chapters in the physical chemistry section and also we have studied about the types of reactions that are exothermic or endothermic reactions.
- Now, let us focus on the potential energy diagram and about the information that can be obtained by this diagram which will help us to obtain the correct answer.
- Basically a potential energy diagram is the graph from which we can illustrate the mechanism for a reaction which is plotted as energy versus the reaction pathway or reaction coordinate.
- Non elementary reaction is the one in which there is no direct correspondence between the stoichiometry and the rate of reaction.
Thus, in the above given graph depicts that the energy of the reactant molecules is lesser than that of the products and hence the reaction is endothermic in nature.
This means that the enthalpy of the formation of products will be higher than the enthalpy of the reactants in the system. Thus the value will be positive, that is $\Delta H$ is positive.
- The reaction that forms hydrogen and nitrogen from ammonia all in the gas state with a $\Delta H = 30.50kJ/mol$
So, the correct answer is “Option B”.
Note: Note that the difference between elementary and non elementary reactions is as follows, elementary reactions are those reactions that are well defined reactions which result from the single collision between two molecules or ions and non elementary reactions consist of a series of the elementary reactions.
Complete Solution :
The concept of the potential energy diagram is familiar to us from the chapters in the physical chemistry section and also we have studied about the types of reactions that are exothermic or endothermic reactions.
- Now, let us focus on the potential energy diagram and about the information that can be obtained by this diagram which will help us to obtain the correct answer.
- Basically a potential energy diagram is the graph from which we can illustrate the mechanism for a reaction which is plotted as energy versus the reaction pathway or reaction coordinate.
- Non elementary reaction is the one in which there is no direct correspondence between the stoichiometry and the rate of reaction.
Thus, in the above given graph depicts that the energy of the reactant molecules is lesser than that of the products and hence the reaction is endothermic in nature.
This means that the enthalpy of the formation of products will be higher than the enthalpy of the reactants in the system. Thus the value will be positive, that is $\Delta H$ is positive.
- The reaction that forms hydrogen and nitrogen from ammonia all in the gas state with a $\Delta H = 30.50kJ/mol$
So, the correct answer is “Option B”.
Note: Note that the difference between elementary and non elementary reactions is as follows, elementary reactions are those reactions that are well defined reactions which result from the single collision between two molecules or ions and non elementary reactions consist of a series of the elementary reactions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




