
Alkyl halides are more reactive than:
(A) vinyl chloride
(B) chlorobenzene
(C) benzyl chloride
(D) All of the above
Answer
587.1k+ views
Hint: Alkyl halides can be converted into alcohol using water or hydroxide as a nucleophile.
This reaction is known as hydrolysis. Hydrolysis is done by aq. $KOH$ moist $A{g_2}O.$
$R - X + KOH\xrightarrow{{boil}}R - OH + KX$
Complete step by step answer:
In the given question vinyl chloride, chlorobenzene, benzyl chloride are given.
Let us discuss hydrolysis of each one by one.
Formula of vinyl chloride is $C{H_2} = CH - Cl$
Chlorine atom is attached to $s{p^2}$ hybridized carbon.
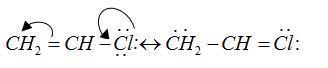
Double bond characters are present so the bond is stronger and difficult to break.
Formula of chlorobenzene is,
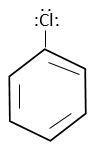
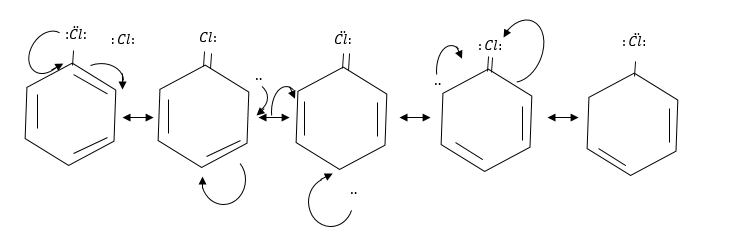
Cl-atom attached to $s{p^2}$ hybridized C-atom and form following resonating structure.
In chlorobenzene double bonds are present between C-atoms and chlorine atoms which are difficult to break.
Benzyl chloride reacts with water in hydrolysis reaction to form benzyl alcohol.
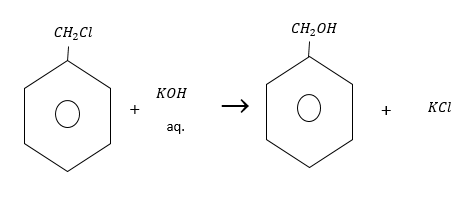
Reactivity of benzyl chloride is less than alkyl chloride.
Therefore, reactivity of alkyl chloride in hydrolysis reactions is highest.
Therefore, from the above explanation is the correct option is (D).
Note:
C-X bond in haloarene and vinyl chloride strangest because carbon atom in this molecule is $s{p^2}$ hybridized and therefore more electronegative than $s{p^3}$ hybridized carbon atom in haloalkane. So $s{p^2}$ hybridized carbon has less tendency to release electrons to the x-atom so C-X bond is less polar in vinyl and aryl halide as compound to alkyl halide.
Cl-atom is attached to $s{p^3}$ hybrid C-atom but C-Cl bond so less reactive than alkyl halide.
This reaction is known as hydrolysis. Hydrolysis is done by aq. $KOH$ moist $A{g_2}O.$
$R - X + KOH\xrightarrow{{boil}}R - OH + KX$
Complete step by step answer:
In the given question vinyl chloride, chlorobenzene, benzyl chloride are given.
Let us discuss hydrolysis of each one by one.
Formula of vinyl chloride is $C{H_2} = CH - Cl$
Chlorine atom is attached to $s{p^2}$ hybridized carbon.
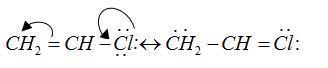
Double bond characters are present so the bond is stronger and difficult to break.
Formula of chlorobenzene is,

Cl-atom attached to $s{p^2}$ hybridized C-atom and form following resonating structure.
In chlorobenzene double bonds are present between C-atoms and chlorine atoms which are difficult to break.

Benzyl chloride reacts with water in hydrolysis reaction to form benzyl alcohol.

Reactivity of benzyl chloride is less than alkyl chloride.
Therefore, reactivity of alkyl chloride in hydrolysis reactions is highest.
Therefore, from the above explanation is the correct option is (D).
Note:
C-X bond in haloarene and vinyl chloride strangest because carbon atom in this molecule is $s{p^2}$ hybridized and therefore more electronegative than $s{p^3}$ hybridized carbon atom in haloalkane. So $s{p^2}$ hybridized carbon has less tendency to release electrons to the x-atom so C-X bond is less polar in vinyl and aryl halide as compound to alkyl halide.
Cl-atom is attached to $s{p^3}$ hybrid C-atom but C-Cl bond so less reactive than alkyl halide.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




