
Aldol condensation product of acetone on dehydration gives:
(a)- But-2-enal
(b)- 2–Methyl-pent–3–en–4–one
(c)- 4–Hydroxy–4–methyl pentan–2 –one
(d)- 4–Methyl–pent–3–en–2–one
Answer
538.2k+ views
Hint: Aldol condensation is a process of addition of molecules in carbonyl compounds, i.e., aldehydes and ketones, this process is only possible if the compound has an alpha-hydrogen atom. On dehydration, there will be the removal of the water molecule, forming a double bond in the compound.
Complete answer:
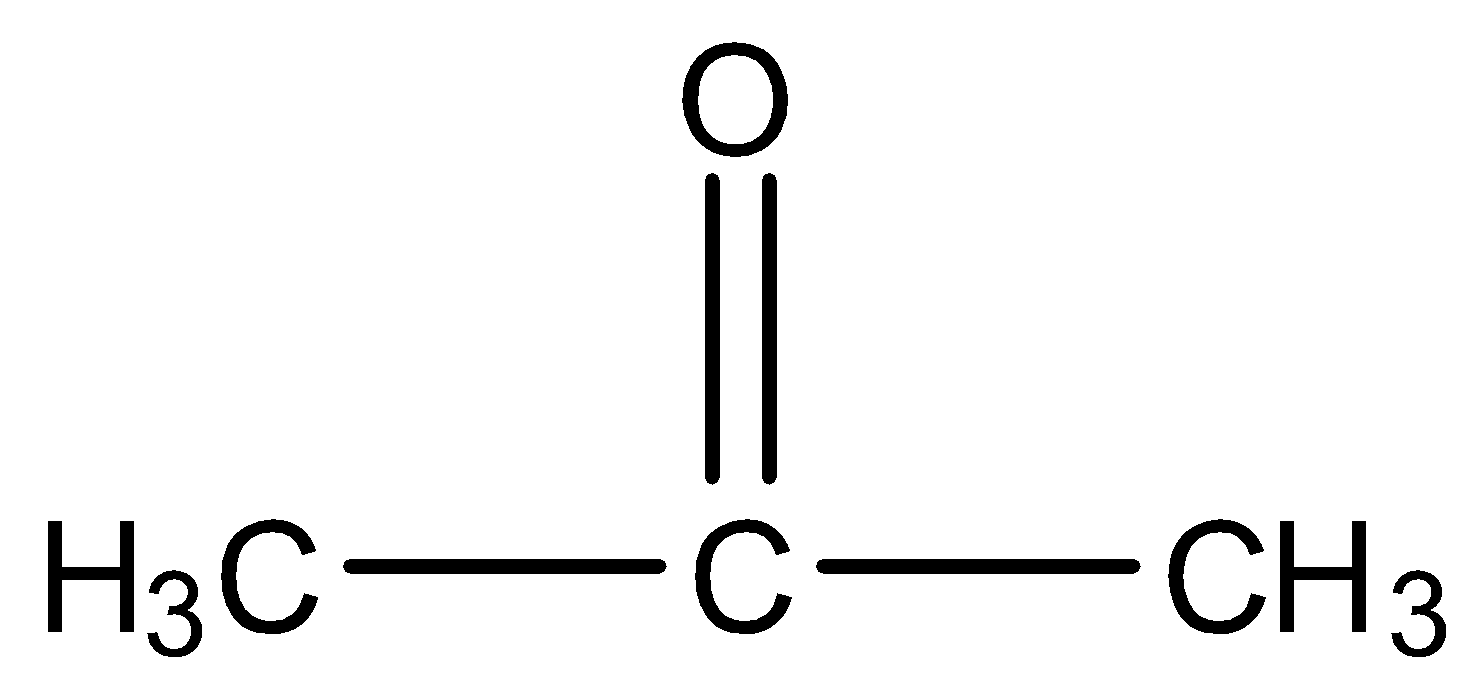
Acetone is a compound having a carbonyl group, i.e., ($>C=O$), or more specifically saying it is a ketone having three carbon atoms and the functional group is at the second carbon atom. The structure is given below:
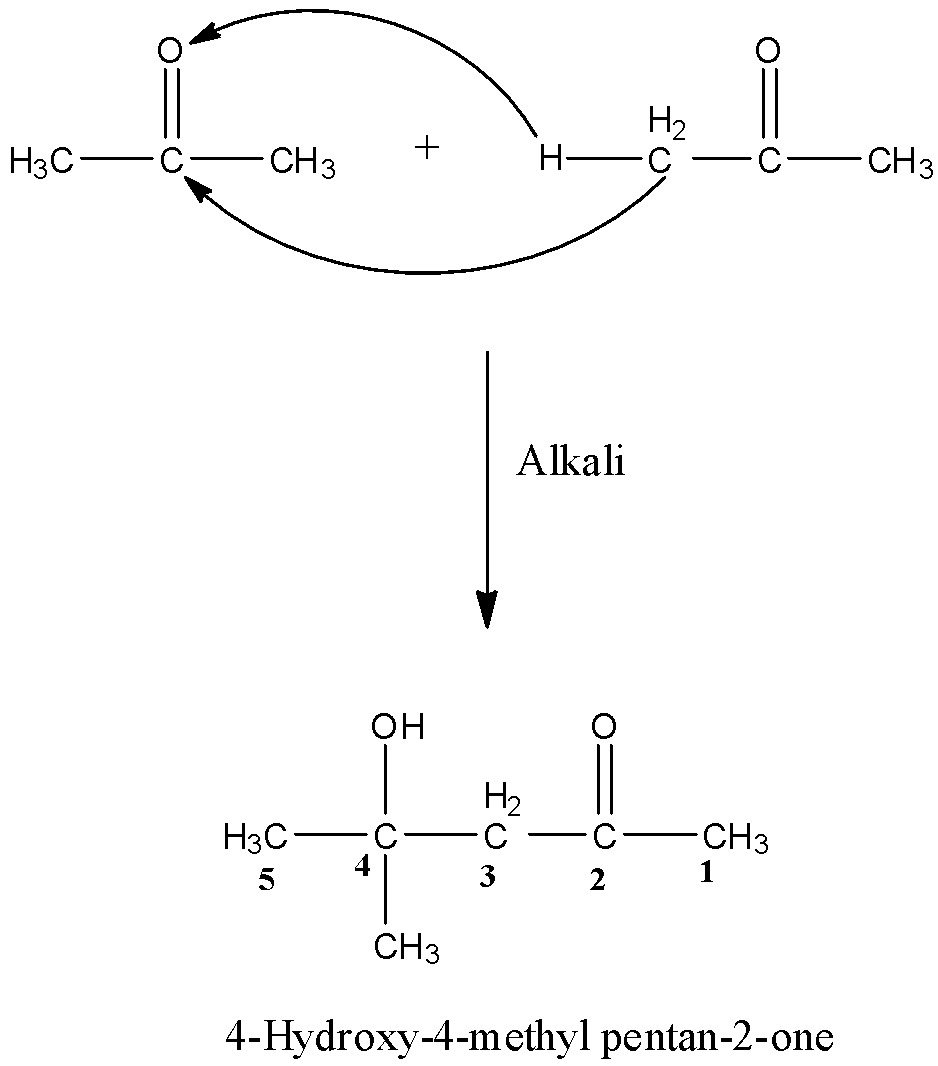
Aldol condensation is a process of the addition of molecules in carbonyl compounds, i.e., aldehydes and ketones, this process is only possible if the compound has an alpha-hydrogen atom. So, when two moles of acetone undergo aldol condensation in the presence of alkali then the alpha-hydrogen of one molecule of acetone will attack the oxygen atom of the other molecule and the rest part will get attach to the carbon atom having the oxygen atom. The reaction is given below:
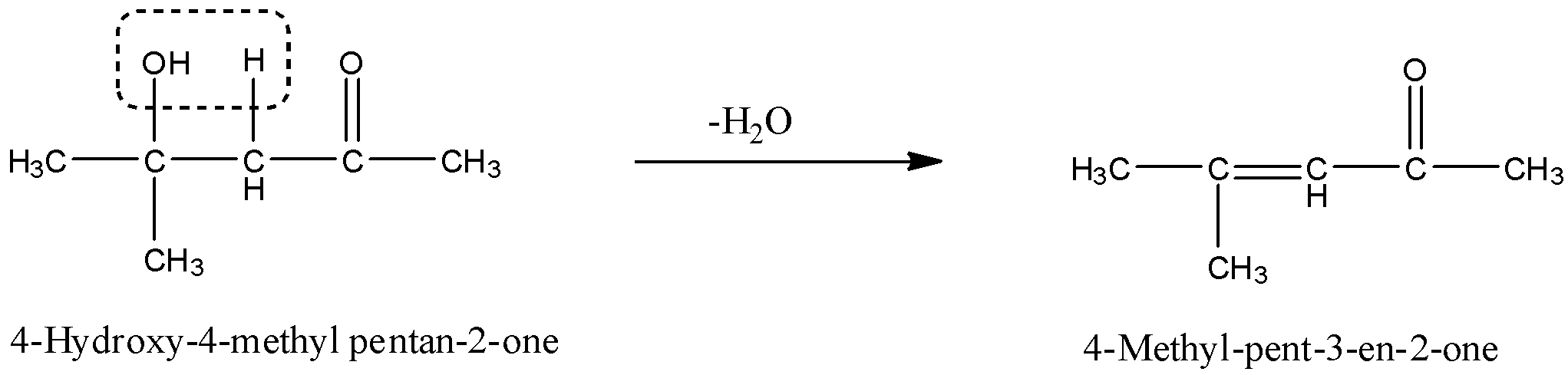
The given product is 4–Hydroxy–4–methyl pentan–2 –one, and when this is dehydrated then there is the removal of a water molecule. The OH from the 4th carbon atom and H from the 3rd carbon atom and will form a double bond. The reaction is given below:
So, the formed product is 4–Methyl–pent–3–en–2–one.
Therefore, the correct answer is an option (d)- 4–Methyl–pent–3–en–2–one.
Note:
The aldol condensation is only possible in the alkaline medium. If two same molecules are there in the reaction, then it is called self-aldol condensation, and if two different molecules are there in the reaction then it is called cross-aldol condensation.
Complete answer:
Acetone is a compound having a carbonyl group, i.e., ($>C=O$), or more specifically saying it is a ketone having three carbon atoms and the functional group is at the second carbon atom. The structure is given below:

Aldol condensation is a process of the addition of molecules in carbonyl compounds, i.e., aldehydes and ketones, this process is only possible if the compound has an alpha-hydrogen atom. So, when two moles of acetone undergo aldol condensation in the presence of alkali then the alpha-hydrogen of one molecule of acetone will attack the oxygen atom of the other molecule and the rest part will get attach to the carbon atom having the oxygen atom. The reaction is given below:

The given product is 4–Hydroxy–4–methyl pentan–2 –one, and when this is dehydrated then there is the removal of a water molecule. The OH from the 4th carbon atom and H from the 3rd carbon atom and will form a double bond. The reaction is given below:

So, the formed product is 4–Methyl–pent–3–en–2–one.
Therefore, the correct answer is an option (d)- 4–Methyl–pent–3–en–2–one.
Note:
The aldol condensation is only possible in the alkaline medium. If two same molecules are there in the reaction, then it is called self-aldol condensation, and if two different molecules are there in the reaction then it is called cross-aldol condensation.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




