
Acyl halide and acid halide are the same groups.
A. True
B. False
Answer
607.5k+ views
Hint- An acid halide is a chemical compound which is derived from an oxoacid by replacing a hydroxyl group with a halide group. and sometimes it is also called as acyl halide If the acid is a carboxylic acid, the compound contains a (–COX) functional group, which consists of a carbonyl group singly bonded to a halogen atom.
Complete answer:
Acyl halide and acid halide are the same groups. They represent the function group.
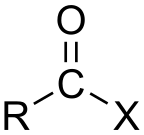
where X is a halogen atom which is Group 17 (Group VII a) of the periodic table i.e. (F, Cl, Br or I). and these are derived from carboxylic acids (which configuration shown below) by replacing a hydroxyl group (-OH) with a halide group.
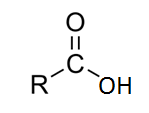
Therefore, from above we understand that,
Acid halides are one example of an acid derivative of carboxylic acid. In this example, the alcohol (-OH) group has been replaced by a chlorine atom; chlorine is the most commonly used acid halide.
For example-

The given statement is True.
Note- Acyl chlorides are the most reactive carboxylic acid derivatives. The electronegative chlorine atom pulls electrons toward it in the C-Cl bond, which makes the carbonyl carbon more electrophilic. This makes nucleophilic attack easier.
Complete answer:
Acyl halide and acid halide are the same groups. They represent the function group.

where X is a halogen atom which is Group 17 (Group VII a) of the periodic table i.e. (F, Cl, Br or I). and these are derived from carboxylic acids (which configuration shown below) by replacing a hydroxyl group (-OH) with a halide group.

Therefore, from above we understand that,
Acid halides are one example of an acid derivative of carboxylic acid. In this example, the alcohol (-OH) group has been replaced by a chlorine atom; chlorine is the most commonly used acid halide.
For example-

The given statement is True.
Note- Acyl chlorides are the most reactive carboxylic acid derivatives. The electronegative chlorine atom pulls electrons toward it in the C-Cl bond, which makes the carbonyl carbon more electrophilic. This makes nucleophilic attack easier.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




