
Acetaldehyde reacts with hydroxyl amine to form _____
A.Formonitrile
B.Acetone cyanohydrin
C.Acetaldoxime
D.Acetoxime
Answer
517.5k+ views
Hint: We have to know that when acetaldehyde reacts with hydroxyl amine it undergoes nucleophilic substitution reaction. In a nucleophilic substitution reaction, the electron rich compound replaces the leaving group. In acetaldehyde, the functional group found is aldehyde. Hydroxylamine is found in crystalline form.
Complete answer:
We know that electrophiles are species which are deficient in electrons, so they have tendency to gain electrons but nucleophiles are species that are rich in electrons, so they have the ability to donate electrons.
We have to know that the reaction between acetaldehyde with hydroxylamine is a nucleophilic substitution reaction. In this nucleophilic substitution reaction, acetaldehyde and hydroxyl amine react to form Acetaldoxime. We can write the chemical reaction as,
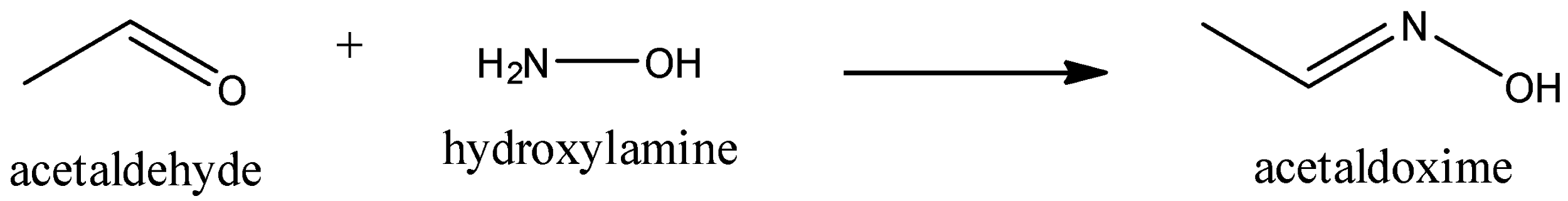
In this reaction, the nucleophilicity found in nitrogen of the hydroxylamine is raised because of the presence of oxygen. Because of the transfer of protons, elimination of water occurs. Mixture of geometric isomers is formed by oxides.
We can draw the skeletal structure of Acetaldoxime is,
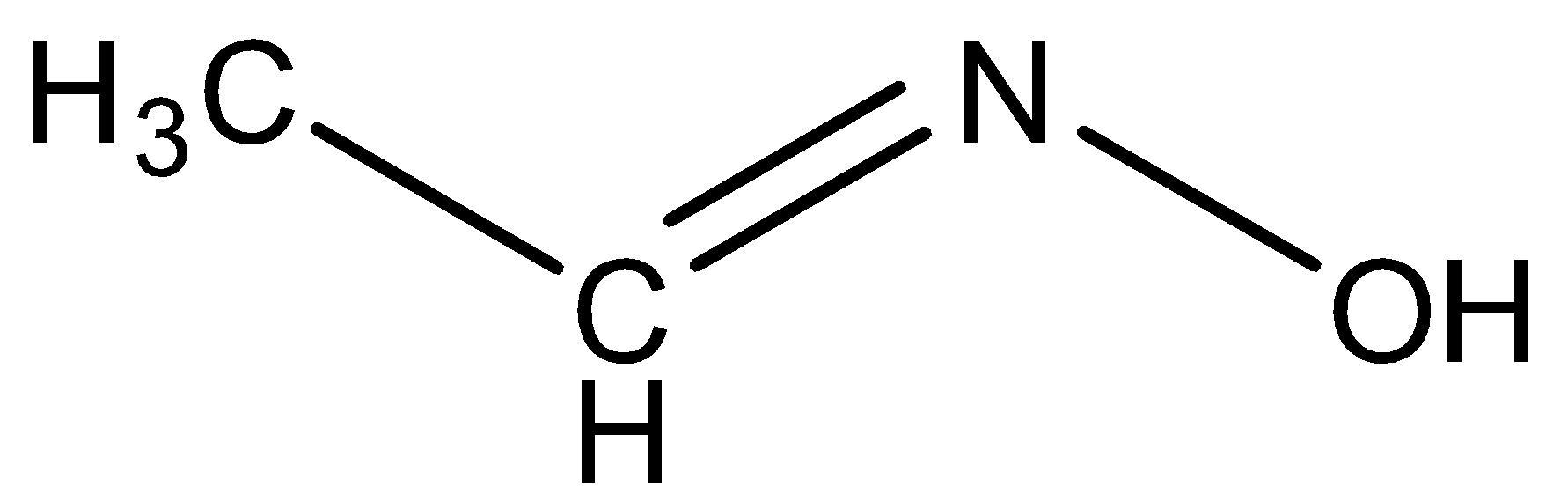
When acetaldehyde reacts with hydroxyl amine by nucleophilic substitution reaction, the product formed is Acetaldoxime. So, we can say that Option (C) is correct.
Note:
We have to know that the reaction between acetaldehyde with hydroxylamine is
condensation reaction. In this reaction, a molecule of acetaldehyde is condensed with hydroxylamine to form Acetaldoxime. As this reaction is a condensation reaction, a molecule of water is eliminated. Hydroxylamine is helpful in the formation of oxides. We have to remember that a compound that contains oxime is Acetaldoxime.
Complete answer:
We know that electrophiles are species which are deficient in electrons, so they have tendency to gain electrons but nucleophiles are species that are rich in electrons, so they have the ability to donate electrons.
We have to know that the reaction between acetaldehyde with hydroxylamine is a nucleophilic substitution reaction. In this nucleophilic substitution reaction, acetaldehyde and hydroxyl amine react to form Acetaldoxime. We can write the chemical reaction as,
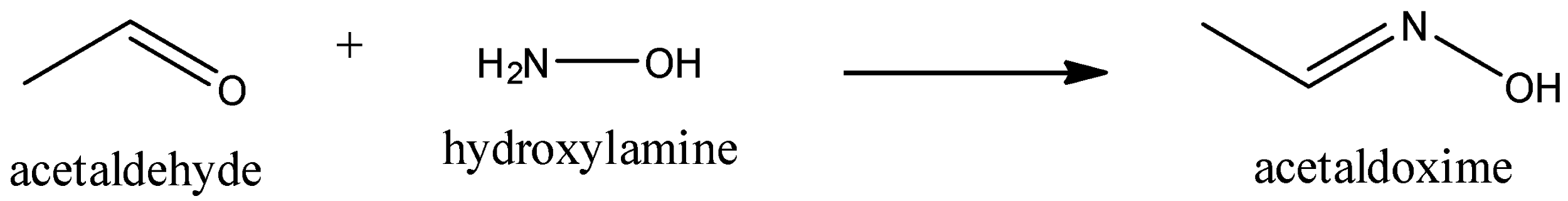
In this reaction, the nucleophilicity found in nitrogen of the hydroxylamine is raised because of the presence of oxygen. Because of the transfer of protons, elimination of water occurs. Mixture of geometric isomers is formed by oxides.
We can draw the skeletal structure of Acetaldoxime is,

When acetaldehyde reacts with hydroxyl amine by nucleophilic substitution reaction, the product formed is Acetaldoxime. So, we can say that Option (C) is correct.
Note:
We have to know that the reaction between acetaldehyde with hydroxylamine is
condensation reaction. In this reaction, a molecule of acetaldehyde is condensed with hydroxylamine to form Acetaldoxime. As this reaction is a condensation reaction, a molecule of water is eliminated. Hydroxylamine is helpful in the formation of oxides. We have to remember that a compound that contains oxime is Acetaldoxime.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




