
Acetaldehyde on reaction with Grignard reagent and subsequent hydrolysis yields:
(a)- Tertiary alcohol
(b)- Primary alcohol
(c)- Secondary alcohol
(d)- Both primary and secondary alcohol
Answer
540.9k+ views
Hint: The given compound in the reaction is acetaldehyde or ethanal and ethanal is a primary compound. Grignard reagent means there is magnesium, alkyl group, and halogen so, its formula will be $RMgX$, when this reacts with carbonyl group then there will be the addition of alkyl group at the carbon atom having the oxygen atom.
Complete answer:
In the reaction given in the question, the reactant is acetaldehyde or ethanal because there are two carbon atoms and there is an aldehyde functional group whose formula will be $C{{H}_{3}}CHO$, and this ethanal reacts with Grignard reagent means there is magnesium, alkyl group, and halogen so, its formula will be $RMgX$. The alkyl group present with MgX in the compound can be methyl, ethyl, propyl, etc.
When this reacts with the carbonyl group then there will be the addition of an alkyl group at the carbon atom having the oxygen atom. Now, when the Acetaldehyde or ethanal reacts with Alkyl magnesium halide, then the alkyl group will attack the carbon atom having the carbonyl group, and the MgX will attack the Oxygen atom.
When this molecule is treated with water then the –OMgX group will be hydrolyzed and it will convert into the –OH group. The series of reaction is given below:
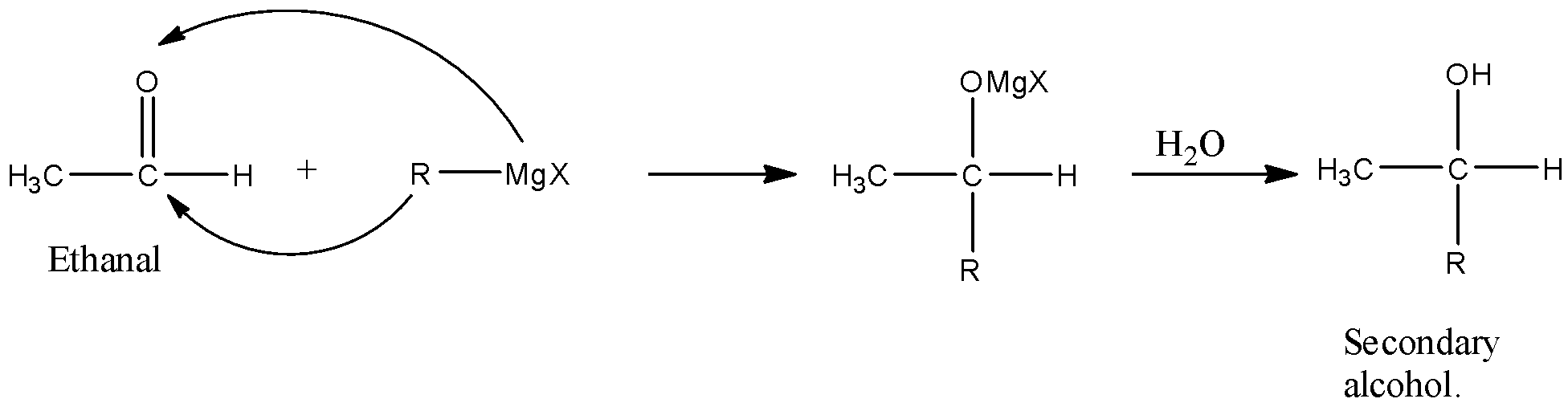
So, the product formed is secondary alcohol because the hydroxyl group is attached with a carbon atom that is further attached with two carbon atoms.
Therefore, the correct answer is an option (c).
Note:
To form primary alcohol by using Grignard reagent then we have to use methanol as reactant because the only methanol can form primary alcohol, and with ketones, always tertiary alcohols are formed.
Complete answer:
In the reaction given in the question, the reactant is acetaldehyde or ethanal because there are two carbon atoms and there is an aldehyde functional group whose formula will be $C{{H}_{3}}CHO$, and this ethanal reacts with Grignard reagent means there is magnesium, alkyl group, and halogen so, its formula will be $RMgX$. The alkyl group present with MgX in the compound can be methyl, ethyl, propyl, etc.
When this reacts with the carbonyl group then there will be the addition of an alkyl group at the carbon atom having the oxygen atom. Now, when the Acetaldehyde or ethanal reacts with Alkyl magnesium halide, then the alkyl group will attack the carbon atom having the carbonyl group, and the MgX will attack the Oxygen atom.
When this molecule is treated with water then the –OMgX group will be hydrolyzed and it will convert into the –OH group. The series of reaction is given below:

So, the product formed is secondary alcohol because the hydroxyl group is attached with a carbon atom that is further attached with two carbon atoms.
Therefore, the correct answer is an option (c).
Note:
To form primary alcohol by using Grignard reagent then we have to use methanol as reactant because the only methanol can form primary alcohol, and with ketones, always tertiary alcohols are formed.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




