
a) Write a short note on Riemer-Tiemann reaction.
b) Two isomeric compounds (A) and (B) have molecular formula ${{C}_{7}}{{H}_{7}}OH$. (A) gives violet colour with neutral ferric chloride solution whereas (B) does not. Suggest structure for (A) and (B).
Answer
585.9k+ views
Hint: Riemer-Tiemann reaction involves the reaction of phenol with chloroform to convert it into salicylaldehyde.
Isomers are compounds with identical molecular formula but different structural formula. Isomers have the same number of each atom but the connectivity of atoms and arrangement of atoms in space is different.
Complete answer:
a) Riemer-Tiemann reaction
Riemer-Tiemann reaction is the reaction used to substitute the ortho-hydrogen in phenol with formyl group ($-CHO$). In this reaction, phenol is treated with chloroform ($CHC{{l}_{3}}$) at 340 K temperature in an aqueous solution of sodium hydroxide (NaOH), the product is hydrolyzed to obtained salicylaldehyde, i.e. o-hydroxy benzaldehyde. The reaction is given below:
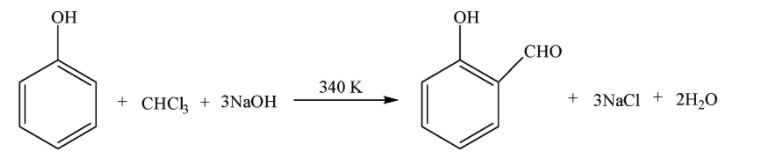
\[\]
Riemer-Tiemann is an electrophilic substitution reaction and involves a carbene intermediate. The mechanism involved in Riemer-Tiemann reaction is shown below:
Generation of electrophile: Dichlorocarbene acts as an electrophile.
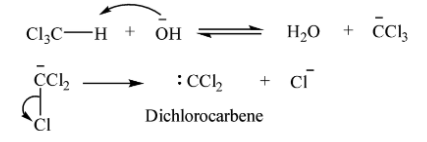
Attack on the electrophile:
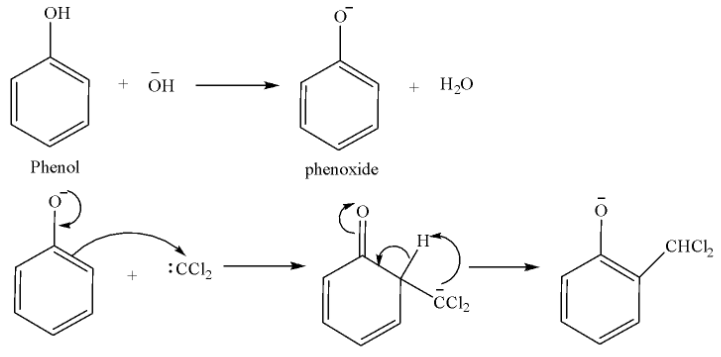
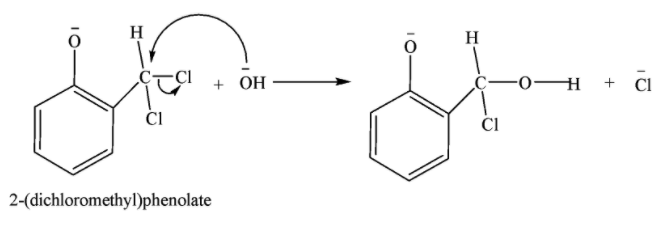
Hydrolysis of substituted phenoxide ion gives salicylaldehyde.
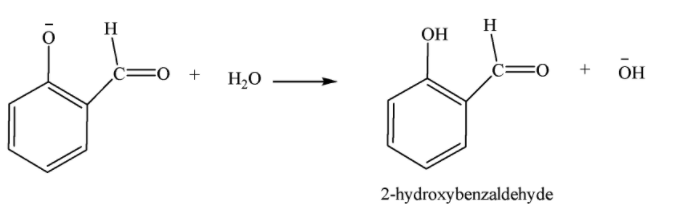
4-hydroxybenzaldehyde is also formed as a minor product.
b) We have been given that (A) and (B) are two isomers having the molecular formula ${{C}_{7}}{{H}_{7}}OH$. Only compound (A) reacts with a neutral solution of ferric chloride ($FeC{{l}_{3}}$) to give violet colour. Let us try to deduce the structure of (A) and (B) with this information.
We know that phenols in addition to ferric chloride give colouration. So, we can conclude that (A) is a phenolic compound.
Molecular formula for phenol is ${{C}_{6}}{{H}_{5}}OH$and the formula given for (A) is ${{C}_{7}}{{H}_{7}}OH$therefore, (A) has to be a substituted phenol.
Molecular formula for a mono-substituted phenol will be ${{C}_{6}}{{H}_{4}}XOH$ where X is a substituent. Difference between the molecular formulas for a substituted phenol and compound (A) is of $C{{H}_{3}}$. This explains that (A) is a methyl substituted phenol. Structure of compound (A) can be any of the following structures given below:
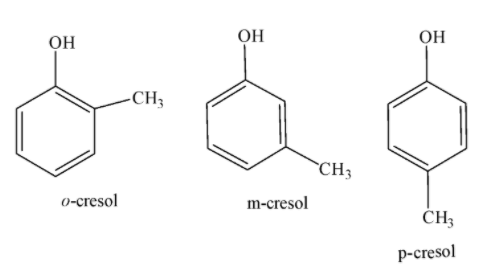
Methyl group may be present at 2, 3 or 4 –position with respect to the hydroxyl group of the phenol.
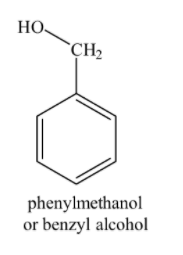
Alcohols do not give colour on reaction with ferric chloride. Therefore, it can be inferred that compound (B) is not a phenol but an alcohol. From the molecular formula given, we can say that it is a phenyl substituted alcohol, i.e. ${{C}_{6}}{{H}_{5}}-C{{H}_{2}}OH$or benzyl alcohol.. Structure for compound (B) is shown below:
Additional information:
Reaction of phenol with ferric chloride forms ferric phenoxide which is responsible for the colour change. This reaction is used in analytical chemistry to distinguish alcohols from phenolic compounds.
Note:
Since all three compounds, i.e. o-cresol, m-cresol and p-cresol give violet colour on reaction with ferric chloride so, it is not possible to decide whether compound (A) is ortho, meta or para substituted methyl phenol with the given information. There is an equal possibility of (A) being any of the three isomers.
Isomers are compounds with identical molecular formula but different structural formula. Isomers have the same number of each atom but the connectivity of atoms and arrangement of atoms in space is different.
Complete answer:
a) Riemer-Tiemann reaction
Riemer-Tiemann reaction is the reaction used to substitute the ortho-hydrogen in phenol with formyl group ($-CHO$). In this reaction, phenol is treated with chloroform ($CHC{{l}_{3}}$) at 340 K temperature in an aqueous solution of sodium hydroxide (NaOH), the product is hydrolyzed to obtained salicylaldehyde, i.e. o-hydroxy benzaldehyde. The reaction is given below:

\[\]
Riemer-Tiemann is an electrophilic substitution reaction and involves a carbene intermediate. The mechanism involved in Riemer-Tiemann reaction is shown below:
Generation of electrophile: Dichlorocarbene acts as an electrophile.

Attack on the electrophile:

Hydrolysis of substituted phenoxide ion gives salicylaldehyde.


4-hydroxybenzaldehyde is also formed as a minor product.
b) We have been given that (A) and (B) are two isomers having the molecular formula ${{C}_{7}}{{H}_{7}}OH$. Only compound (A) reacts with a neutral solution of ferric chloride ($FeC{{l}_{3}}$) to give violet colour. Let us try to deduce the structure of (A) and (B) with this information.
We know that phenols in addition to ferric chloride give colouration. So, we can conclude that (A) is a phenolic compound.
Molecular formula for phenol is ${{C}_{6}}{{H}_{5}}OH$and the formula given for (A) is ${{C}_{7}}{{H}_{7}}OH$therefore, (A) has to be a substituted phenol.
Molecular formula for a mono-substituted phenol will be ${{C}_{6}}{{H}_{4}}XOH$ where X is a substituent. Difference between the molecular formulas for a substituted phenol and compound (A) is of $C{{H}_{3}}$. This explains that (A) is a methyl substituted phenol. Structure of compound (A) can be any of the following structures given below:

Methyl group may be present at 2, 3 or 4 –position with respect to the hydroxyl group of the phenol.
Alcohols do not give colour on reaction with ferric chloride. Therefore, it can be inferred that compound (B) is not a phenol but an alcohol. From the molecular formula given, we can say that it is a phenyl substituted alcohol, i.e. ${{C}_{6}}{{H}_{5}}-C{{H}_{2}}OH$or benzyl alcohol.. Structure for compound (B) is shown below:

Additional information:
Reaction of phenol with ferric chloride forms ferric phenoxide which is responsible for the colour change. This reaction is used in analytical chemistry to distinguish alcohols from phenolic compounds.
Note:
Since all three compounds, i.e. o-cresol, m-cresol and p-cresol give violet colour on reaction with ferric chloride so, it is not possible to decide whether compound (A) is ortho, meta or para substituted methyl phenol with the given information. There is an equal possibility of (A) being any of the three isomers.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




