
(A) What are ionic compounds? Show the formation of magnesium oxide.
(B) Among Covalent and ionic compounds which will have higher melting and boiling point and why?
Answer
577.8k+ views
Hint: Ionic compounds are composed of ions which are formed by loss or gain of electrons. Structure of magnesium oxide can be shown by drawing Lewis Dot structure.
Covalent compounds have weak van Der Waals intermolecular force of attraction.
Complete answer:
As we know that Ions are formed by a loss or gain of electrons. The ion formed by an atom depends upon the atomic structure and number of valence electrons of that atom. Ionic compounds are formed when there is a transfer of electrons between the atoms.
An ionic compound is made up of ions held together by a strong electrostatic force called ionic bonding. The ionic compound is neutral overall, but it consists of positively charged ions(also known as cations) and negatively charged ions(also known as anions).
As we can see, Magnesium has 2 extra electrons, whereas oxygen has 2 less electrons to attain noble gas electronic configuration.
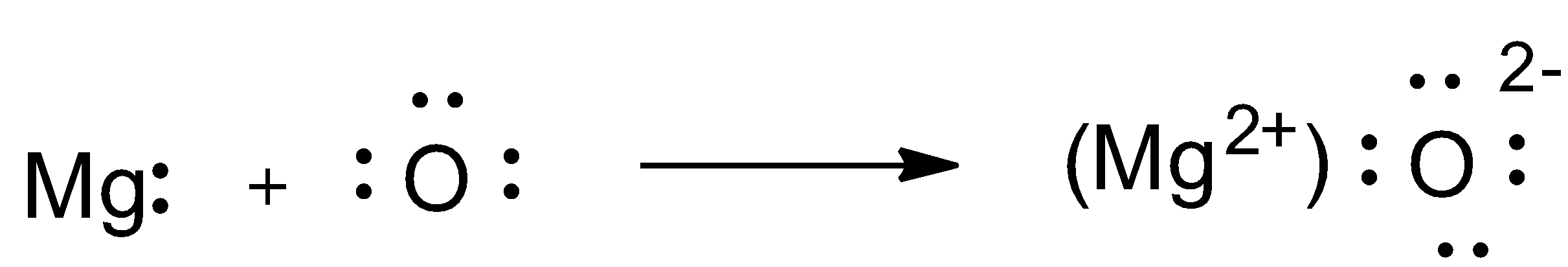
Magnesium forms $M{{g}^{2+}}$ by losing 2 electrons and attains stable noble gas electronic configuration.
Oxygen accepts these 2 electrons from magnesium and form ${{O}^{2-}}$ and attains stable noble gas electronic configuration and hence form $MgO$ ionic compound.
(B) Among Covalent and ionic compounds, ionic compounds will have higher melting and boiling point.
Covalent bonds are formed by the sharing of electrons between two or more atoms.Two atoms with similar electronegativity do not exchange an electron from their outermost shell, the atoms instead they share electrons so that their valence electron shell is completely filled.
Whereas Ionic bonding occurs when there is a large difference in electronegativity between two atoms. Consequently, this large difference in electronegativity leads to a loss of an electron from the less electronegative atom and the gain of that electron by the more electronegative atom, resulting in two ions. These oppositely charged ions feel an attraction to each other, and this electrostatic attraction constitutes an ionic bond and forms ionic compounds.
Ionic compounds have high melting and boiling points because there is a strong intermolecular as well as intramolecular electrostatic force of attraction between the oppositely charged ions and hence a large amount of energy is needed to break the strong bonding force between ions.
Covalent bonds,on the other hand, have weak van der Waals intermolecular forces.as a result it becomes easy to move individual molecules away from other molecules as the bonding between molecules is weak, hence it needs little energy to break. Hence have low melting and boiling point as compared to ionic compounds.
Additional information:
Covalent bonds mostly occur between nonmetals or between two of the similar elements. Covalent compounds have van der Waals intermolecular forces that form bonds of various strengths with other covalent compounds. The three types of van der Waals forces are- 1) dispersion (weak), 2) dipole-dipole (medium), and 3) hydrogen (strong).
Note:
Practically, purely ionic bonding cannot exist, because the vicinity of the species involved in the bonding allows some degree of sharing electron density between them. Hence, all ionic bonding has some covalent character. Bonding is considered ionic when the ionic character is greater than the covalent character and vice-versa. The greater the difference in electronegativity between the two types of atoms involved in the bonding, the more ionic (polar) character it has.also, Bonds with partially ionic and partially covalent character are known as polar covalent bonds.
Covalent compounds have weak van Der Waals intermolecular force of attraction.
Complete answer:
As we know that Ions are formed by a loss or gain of electrons. The ion formed by an atom depends upon the atomic structure and number of valence electrons of that atom. Ionic compounds are formed when there is a transfer of electrons between the atoms.
An ionic compound is made up of ions held together by a strong electrostatic force called ionic bonding. The ionic compound is neutral overall, but it consists of positively charged ions(also known as cations) and negatively charged ions(also known as anions).
| Element | Symbol | Atomic number | Electronic configuration in shells K,L,M | No. of outermost electrons |
| Magnesium | Mg | 12 | 2,8,2 | 2 |
| Oxygen | O | 8 | 2,6 | 6 |
As we can see, Magnesium has 2 extra electrons, whereas oxygen has 2 less electrons to attain noble gas electronic configuration.

Magnesium forms $M{{g}^{2+}}$ by losing 2 electrons and attains stable noble gas electronic configuration.
Oxygen accepts these 2 electrons from magnesium and form ${{O}^{2-}}$ and attains stable noble gas electronic configuration and hence form $MgO$ ionic compound.
(B) Among Covalent and ionic compounds, ionic compounds will have higher melting and boiling point.
Covalent bonds are formed by the sharing of electrons between two or more atoms.Two atoms with similar electronegativity do not exchange an electron from their outermost shell, the atoms instead they share electrons so that their valence electron shell is completely filled.
Whereas Ionic bonding occurs when there is a large difference in electronegativity between two atoms. Consequently, this large difference in electronegativity leads to a loss of an electron from the less electronegative atom and the gain of that electron by the more electronegative atom, resulting in two ions. These oppositely charged ions feel an attraction to each other, and this electrostatic attraction constitutes an ionic bond and forms ionic compounds.
Ionic compounds have high melting and boiling points because there is a strong intermolecular as well as intramolecular electrostatic force of attraction between the oppositely charged ions and hence a large amount of energy is needed to break the strong bonding force between ions.
Covalent bonds,on the other hand, have weak van der Waals intermolecular forces.as a result it becomes easy to move individual molecules away from other molecules as the bonding between molecules is weak, hence it needs little energy to break. Hence have low melting and boiling point as compared to ionic compounds.
Additional information:
Covalent bonds mostly occur between nonmetals or between two of the similar elements. Covalent compounds have van der Waals intermolecular forces that form bonds of various strengths with other covalent compounds. The three types of van der Waals forces are- 1) dispersion (weak), 2) dipole-dipole (medium), and 3) hydrogen (strong).
Note:
Practically, purely ionic bonding cannot exist, because the vicinity of the species involved in the bonding allows some degree of sharing electron density between them. Hence, all ionic bonding has some covalent character. Bonding is considered ionic when the ionic character is greater than the covalent character and vice-versa. The greater the difference in electronegativity between the two types of atoms involved in the bonding, the more ionic (polar) character it has.also, Bonds with partially ionic and partially covalent character are known as polar covalent bonds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




