
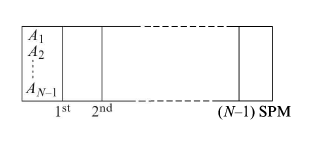
A very long rectangular box is divided into $ N $ equal compartments with $ \left( N-1 \right) $ fixed semipermeable in only first compartment and they can pass through only those SPM whose number is less than or membranes (SPM) numbered from $ 1 $ to $ \left( N-1 \right) $ as shown in the figure. The gases are initially present equal to their subscript. For example, gas $ A1 $ can pass through only the first SPM; gas $ A2 $ can pass only through the first and second SPM, and so on. If initially all gases have the same moles and after a long time, the ratio of partial pressures of gas $ A4 $ in the third compartment to that of gas $ AN-1 $ in first compartment is $ 3, $ the value of $ N $ is?

Answer
539.4k+ views
Hint :We know that the Dalton's Law of Partial Pressures states that the total Pressure exerted by the mixture of gases is equal to the sum of Partial Pressure of each individual Gas present. Each gas is assumed to be an ideal gas and given by formula $ \dfrac{P{{A}_{4}}}{P{{A}_{N-1}}}=\dfrac{n/5}{n/N}=3 $
Complete step by step solution:
The mole fraction of a specific gas in a mixture of gases is equal to the ratio of the partial pressure of that gas to the total pressure exerted by the gaseous mixture. This mole fraction can also be used to calculate the total number of moles of a constituent gas when the total number of moles in the mixture is known
Since $ A1 $ can pass only through first $ 5Pm $
i.e. $ A1 $ will be present in first & second compartment which is given by $ A2 $ will be in $ 1,2,3 $ and $ A3 $ in $ 1,2,3,4 $
and so on we earlier got the fraction value $ \dfrac{P{{A}_{4}}}{P{{A}_{N-1}}}=\dfrac{n/5}{n/N}=3 $
By taking denominator in numerator we get;
$ \dfrac{P{{A}_{4}}}{P{{A}_{N-1}}}=\dfrac{n}{5}\times \dfrac{N}{n}=3 $
Thus we get
$ \dfrac{P{{A}_{4}}}{P{{A}_{N-1}}}=\dfrac{N}{5}=3 $ i.e. $ \Rightarrow \dfrac{N}{5}=3 $
Note :
Note that the applications of Dalton's partial pressures definition in the determination of pressure of a dry gas .In the calculation of partial pressure, expressing Partial Pressures in Terms of Mole Diffraction: the volume occupied by a specific gas in a mixture can also be calculated with this mole diffraction.
Complete step by step solution:
The mole fraction of a specific gas in a mixture of gases is equal to the ratio of the partial pressure of that gas to the total pressure exerted by the gaseous mixture. This mole fraction can also be used to calculate the total number of moles of a constituent gas when the total number of moles in the mixture is known
Since $ A1 $ can pass only through first $ 5Pm $
i.e. $ A1 $ will be present in first & second compartment which is given by $ A2 $ will be in $ 1,2,3 $ and $ A3 $ in $ 1,2,3,4 $
and so on we earlier got the fraction value $ \dfrac{P{{A}_{4}}}{P{{A}_{N-1}}}=\dfrac{n/5}{n/N}=3 $
By taking denominator in numerator we get;
$ \dfrac{P{{A}_{4}}}{P{{A}_{N-1}}}=\dfrac{n}{5}\times \dfrac{N}{n}=3 $
Thus we get
$ \dfrac{P{{A}_{4}}}{P{{A}_{N-1}}}=\dfrac{N}{5}=3 $ i.e. $ \Rightarrow \dfrac{N}{5}=3 $
| $ A1=\dfrac{n}{2} $ | $ A1=\dfrac{n}{2} $ | $ A2=\dfrac{n}{3} $ |
| $ A2=\dfrac{n}{3} $ | $ A2=\dfrac{n}{3} $ | $ A3=\dfrac{n}{4} $ |
| $ A3=\dfrac{n}{4} $ | $ A3=\dfrac{n}{4} $ | $ A3=\dfrac{n}{4} $ |
Note :
Note that the applications of Dalton's partial pressures definition in the determination of pressure of a dry gas .In the calculation of partial pressure, expressing Partial Pressures in Terms of Mole Diffraction: the volume occupied by a specific gas in a mixture can also be calculated with this mole diffraction.
Recently Updated Pages
Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 Physics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




