
a. The hydrogen atom of chloroform is acidic. Explain.
b. Why is dehydrohalogenation reaction in haloalkanes terms as Beta-elimination reaction?
Answer
573k+ views
Hint: Chloroform is a substance that has a strong non- irritating smell. It was used a s an anaesthetic in the old times but was discontinued due to deaths it caused. Chloroform acts as a Lewis acid due to the presence of the acidic hydrogen. The hydrogen is acidic due to electronegativity differences between \[Cl\] atoms and it.
Dehydrohalogenation of haloalkanes is a β-elimination reaction as the hydrogen eliminated along with halogen is at the \[\beta \]- position.
Complete step by step solution:
(a) The hydrogen in chloroform is acidic and dehydrohalogenation reaction in haloalkanes is termed beta-elimination reaction. We have to give a reason why this occurs.
Hydrogen atoms of chloroform are acidic due to three electronegative chlorine atoms present on carbon. The latter requires a partial positive charge due to the \[-{\text{ }}I\] effect of chlorine with the result it tends to attract electrons to the \[C - H\] bonds towards itself. So the removal of the hydrogen atom as a proton becomes easy.
The structure of Chloroform is:
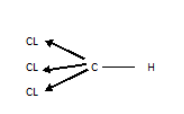
Since the cl attract the shared pair of electrons of the \[C - Cl\] bond, there is a partial \[ + ve\] charge on carbon, and as you know \[C\] is a little more electronegative than \[H\] , it in turn tries to pull the electrons from H. The presence of \[3 - Cl\] groups aggravates the pull. Also CL accepts the lone pair left behind by \[H\] and stabilizes the \[-ve\] charge on \[C\]. thus chloroform behaves as an acid.
(b) In elimination reactions some molecules leave the reactant or are eliminated to form a double or triple bond. In a carbon chain, the carbon which carries the functional group is termed as \[\alpha - \]carbon while the carbon next to it is termed as \[\beta \]- carbon. The hydrogen attached to the \[\beta \]- carbon is termed as \[\beta \]- hydrogen.
In dehydrohalogenation of haloalkanes, alcohol \[KOH\] is used. When alkyl halides have \[\beta \]- hydrogen are heated with alc. \[KOH\], the halogen is eliminated from \[\alpha - \]position and hydrogen from \[\beta \]-position leading to formation of alkenes.
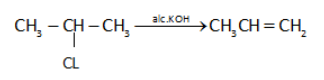
This reaction is called \[\beta \]-elimination because the relative position of eliminated substituents is 1,2 (and hydrogen is eliminated from \[\beta \]-position).
Note: Students must go through the steps to understand the concept. Also learning about the \[-{\text{ }}I\] effect, \[ + I\] effect, \[ + R/ - R\] effect, etc is helpful in understanding the chemistry behind it. Also one must not confuse between elimination, addition, substitution and rearrangement reactions.
Dehydrohalogenation of haloalkanes is a β-elimination reaction as the hydrogen eliminated along with halogen is at the \[\beta \]- position.
Complete step by step solution:
(a) The hydrogen in chloroform is acidic and dehydrohalogenation reaction in haloalkanes is termed beta-elimination reaction. We have to give a reason why this occurs.
Hydrogen atoms of chloroform are acidic due to three electronegative chlorine atoms present on carbon. The latter requires a partial positive charge due to the \[-{\text{ }}I\] effect of chlorine with the result it tends to attract electrons to the \[C - H\] bonds towards itself. So the removal of the hydrogen atom as a proton becomes easy.
The structure of Chloroform is:

Since the cl attract the shared pair of electrons of the \[C - Cl\] bond, there is a partial \[ + ve\] charge on carbon, and as you know \[C\] is a little more electronegative than \[H\] , it in turn tries to pull the electrons from H. The presence of \[3 - Cl\] groups aggravates the pull. Also CL accepts the lone pair left behind by \[H\] and stabilizes the \[-ve\] charge on \[C\]. thus chloroform behaves as an acid.
(b) In elimination reactions some molecules leave the reactant or are eliminated to form a double or triple bond. In a carbon chain, the carbon which carries the functional group is termed as \[\alpha - \]carbon while the carbon next to it is termed as \[\beta \]- carbon. The hydrogen attached to the \[\beta \]- carbon is termed as \[\beta \]- hydrogen.
In dehydrohalogenation of haloalkanes, alcohol \[KOH\] is used. When alkyl halides have \[\beta \]- hydrogen are heated with alc. \[KOH\], the halogen is eliminated from \[\alpha - \]position and hydrogen from \[\beta \]-position leading to formation of alkenes.
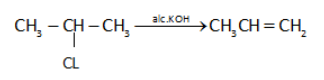
This reaction is called \[\beta \]-elimination because the relative position of eliminated substituents is 1,2 (and hydrogen is eliminated from \[\beta \]-position).
Note: Students must go through the steps to understand the concept. Also learning about the \[-{\text{ }}I\] effect, \[ + I\] effect, \[ + R/ - R\] effect, etc is helpful in understanding the chemistry behind it. Also one must not confuse between elimination, addition, substitution and rearrangement reactions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




