
A system changes form state X to Y with a change in internal energy measuring to 25KJ/mol, by a reversible path and returns form Y to X by an irreversible path, what will be the net change in internal energy?
A) 25 KJ
B) > 25KJ
C) < 25 KJ
D) Zero
Answer
590.1k+ views
Hint: Internal energy is the sum of potential energy and the kinetic energy of the total reaction. The change in internal energy (\[\Delta U\]) of a reaction is equal to the enthalpy change in a reaction, when the reaction is carried out at constant pressure.
Complete answer:
In the question it is mentioned that a system changes form state X to Y by a reversible path and returns form Y to X by an irreversible path means it is a cyclic process.
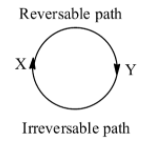
We know that in a cyclic process the net change in internal energy (\[\Delta U\]) is equal to zero and the change in the internal energy of the reaction does not depend on the path by which the final state of the chemical is reached.
Internal energy is a state function and it is not dependent on the path.
Therefore the net change in the internal energy for a cyclic process is zero.
So, the correct option is D.
Note: The internal energy in a cyclic process is not changed because the system returns to its initial state after completion of the cycle. The heat involved in the system equals the work done by the system in a cyclic process.
The enthalpy (H) of a reaction is defined as the sum of internal energy (U) and the work required to attain its pressure (P) and volume (V).
H = U + PV,
Enthalpy is directly proportional to internal energy of the reaction.
Complete answer:
In the question it is mentioned that a system changes form state X to Y by a reversible path and returns form Y to X by an irreversible path means it is a cyclic process.

We know that in a cyclic process the net change in internal energy (\[\Delta U\]) is equal to zero and the change in the internal energy of the reaction does not depend on the path by which the final state of the chemical is reached.
Internal energy is a state function and it is not dependent on the path.
Therefore the net change in the internal energy for a cyclic process is zero.
So, the correct option is D.
Note: The internal energy in a cyclic process is not changed because the system returns to its initial state after completion of the cycle. The heat involved in the system equals the work done by the system in a cyclic process.
The enthalpy (H) of a reaction is defined as the sum of internal energy (U) and the work required to attain its pressure (P) and volume (V).
H = U + PV,
Enthalpy is directly proportional to internal energy of the reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




