
A sample of gas has been found to be expanding from volume ${{V}_{1}}$ to ${{V}_{2}}$ . The amount of work done by the gas will be the greatest when the expansion has,
A. isothermal
B. isobaric
C. adiabatic
D. similar in all case
Answer
569.7k+ views
Hint: An isobaric process will be defined as the process in thermodynamics in which the pressure will remain fixed. An adiabatic process can be defined as a type of thermodynamic process which will be happening without the transfer of heat or mass between the system and its surroundings. An isothermal process can be defined as the type of process in thermodynamics in which the temperature of the system remains fixed. This will help you in answering this question.
Complete answer:
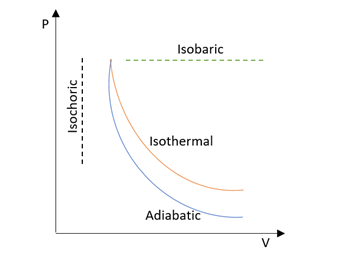
An isobaric process will be defined as the thermodynamic process in which the pressure will remain fixed. This will be generally reached by permitting the volume in order to expand or contract to neutralize any pressure variation that would be caused by the heat transfer. An isothermal process can be defined as the type of process in thermodynamics in which the temperature of the system remains fixed. An adiabatic process can be defined as a type of thermodynamic process which will be happening without the transfer of heat or mass between the system and its surroundings. An adiabatic process unlike an isothermal process will transfer the energy to the surroundings only as work. At the fixed pressure, the PV curve will be enclosing the maximum area. Hence the work will be maximum in such a case compared to any other processes.
Therefore the correct answer has been obtained as option B.
Note:
It will be a process where there will be a gas compression and heat is produced. One of the simplest examples will be the release of the air from a pneumatic tire. Adiabatic Efficiency will be applied to the devices like the compressors, and turbines.
Complete answer:
An isobaric process will be defined as the thermodynamic process in which the pressure will remain fixed. This will be generally reached by permitting the volume in order to expand or contract to neutralize any pressure variation that would be caused by the heat transfer. An isothermal process can be defined as the type of process in thermodynamics in which the temperature of the system remains fixed. An adiabatic process can be defined as a type of thermodynamic process which will be happening without the transfer of heat or mass between the system and its surroundings. An adiabatic process unlike an isothermal process will transfer the energy to the surroundings only as work. At the fixed pressure, the PV curve will be enclosing the maximum area. Hence the work will be maximum in such a case compared to any other processes.
Therefore the correct answer has been obtained as option B.

Note:
It will be a process where there will be a gas compression and heat is produced. One of the simplest examples will be the release of the air from a pneumatic tire. Adiabatic Efficiency will be applied to the devices like the compressors, and turbines.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




