
What is a racemic mixture? Give an example.
Answer
567.6k+ views
Hint: Optically active compounds behave differently in plane polarized light. There were some compounds which were found to be optically active but did not behave differently in plane polarized light. This led to the study of those compounds and the reason for the behaviour was deduced.
Complete step by step solution:
-The carbon atom with 4 different groups associated with it is called chiral carbon atom and the compound is optically active, thus rotates plane polarised light in clockwise and anti-clockwise direction.
-When 2 carbon atoms in a compound are chiral, the resulting molecule can either be diastereomer or enantiomer. Enantiomers are the isomers which are non-superimposable mirror images of each other. Diastereomers are the stereoisomers which are not the mirror images of each other.
-Diastereomers have different physical and chemical properties and so can easily be separated.
Enantiomers have identical physical properties and chemical properties if they react with achiral molecules but different properties if they react with chiral molecules.
-But if we take equimolar concentration of pure dextro(d) and pure leavo(l) form of an optically active compound, then the mixture thus formed is the racemic mixture.
-Racemic mixture is optically inactive. It is because one form rotates the plane polarised light in one direction while the other form rotates it in the opposite direction. Thus the effect gets cancelled out. This is called EXTERNAL COMPENSATION.
-If there are an equal number of dextrorotatory and leavorotating molecules, the net optical rotation of the racemate is then considered to be zero.
-Racemic mixtures have their significance as they can be used to separate the optically active isomers from one another which is otherwise impossible in case of enantiomers. The process of separation of racemic mixture into its optically active pure dextro and pure leavo form is called resolution.
Eg.
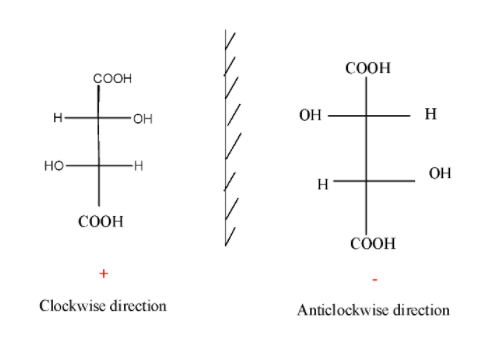
Note: If the rotation of a compound in presence of plane polarized light occurs in clockwise direction, it is called dextro and is denoted by d. If the rotation of a compound in presence of plane polarized light occurs in an anti-clockwise direction, it is called leavo and is denoted by l.
Complete step by step solution:
-The carbon atom with 4 different groups associated with it is called chiral carbon atom and the compound is optically active, thus rotates plane polarised light in clockwise and anti-clockwise direction.
-When 2 carbon atoms in a compound are chiral, the resulting molecule can either be diastereomer or enantiomer. Enantiomers are the isomers which are non-superimposable mirror images of each other. Diastereomers are the stereoisomers which are not the mirror images of each other.
-Diastereomers have different physical and chemical properties and so can easily be separated.
Enantiomers have identical physical properties and chemical properties if they react with achiral molecules but different properties if they react with chiral molecules.
-But if we take equimolar concentration of pure dextro(d) and pure leavo(l) form of an optically active compound, then the mixture thus formed is the racemic mixture.
-Racemic mixture is optically inactive. It is because one form rotates the plane polarised light in one direction while the other form rotates it in the opposite direction. Thus the effect gets cancelled out. This is called EXTERNAL COMPENSATION.
-If there are an equal number of dextrorotatory and leavorotating molecules, the net optical rotation of the racemate is then considered to be zero.
-Racemic mixtures have their significance as they can be used to separate the optically active isomers from one another which is otherwise impossible in case of enantiomers. The process of separation of racemic mixture into its optically active pure dextro and pure leavo form is called resolution.
Eg.

Note: If the rotation of a compound in presence of plane polarized light occurs in clockwise direction, it is called dextro and is denoted by d. If the rotation of a compound in presence of plane polarized light occurs in an anti-clockwise direction, it is called leavo and is denoted by l.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




