
A phase diagram for solvents and solutions is shown in the figure. What represents the normal boiling point of the solution?

Answer
515.4k+ views
Hint: We are discussing the normal boiling point of the solution. We should know first what the normal boiling point is. Normal boiling point of a solution is the temperature at which the vapour pressure of the solution becomes equal to the atmospheric pressure. We are given the phase diagram for solvents and solutions. We will study the diagram to solve this question.
Complete answer:
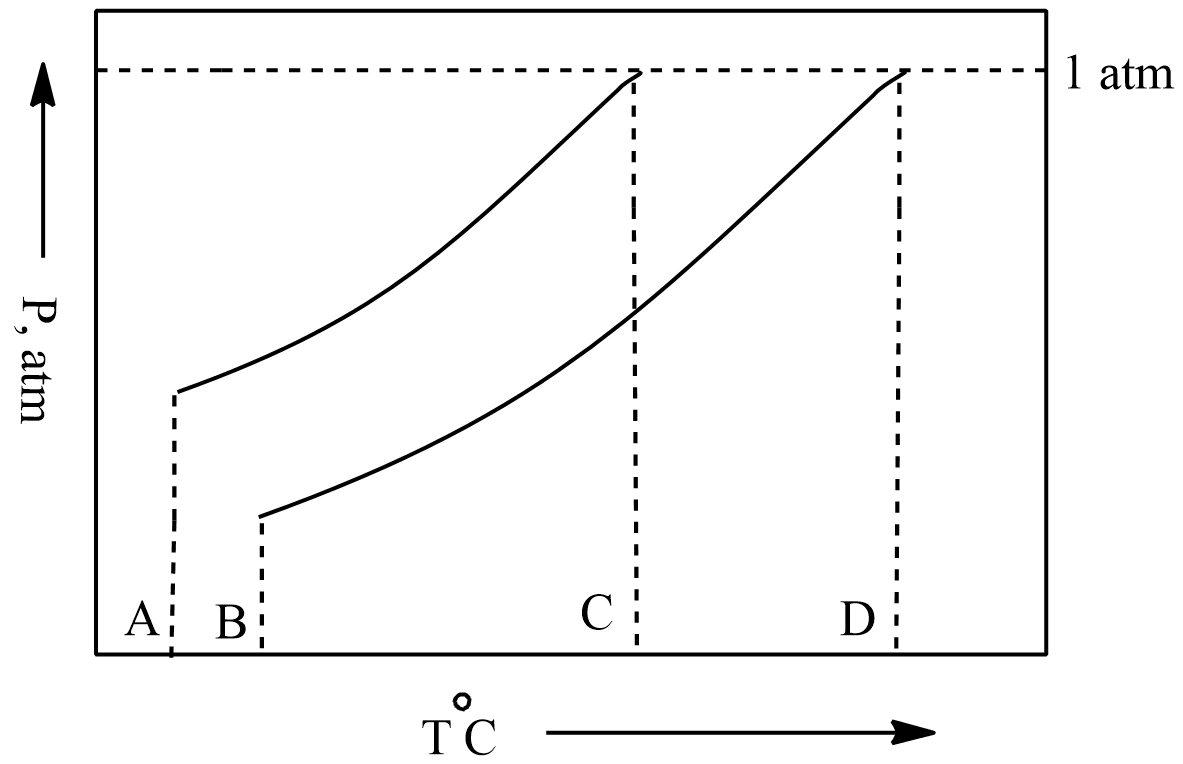
Phase diagrams are pressure vs temperature which is used to predict the state of the solvent and the solution.
Point A in the phase diagram is the initial state of solvent showing the temperature and pressure at that time.
Now when we see point B is the initial state of the solution, we can see that when we add any solute to pure solvent its pressure is decreased which can be seen in the phase diagram as pressure at point A was higher because it is of pure solvent.
Now we move on to point C. It is the state where the vapour pressure of the solvent becomes equal to the atmospheric pressure so point C represents the Boiling point of the solvent.
Now we are at the last point D. It is the state where the vapour pressure of the solution becomes equal to atmospheric pressure, so point D represents the normal boiling point of the solution.
So, Point D is the correct answer.
Note:
The difference between the Point D and Point C i.e., boiling point of the solution and boiling point of the solvent is known as the elevation in boiling point. It can be observed from the phase diagram that the boiling point of solution is higher than the boiling point of pure solvent.
Complete answer:
Phase diagrams are pressure vs temperature which is used to predict the state of the solvent and the solution.
Point A in the phase diagram is the initial state of solvent showing the temperature and pressure at that time.
Now when we see point B is the initial state of the solution, we can see that when we add any solute to pure solvent its pressure is decreased which can be seen in the phase diagram as pressure at point A was higher because it is of pure solvent.
Now we move on to point C. It is the state where the vapour pressure of the solvent becomes equal to the atmospheric pressure so point C represents the Boiling point of the solvent.
Now we are at the last point D. It is the state where the vapour pressure of the solution becomes equal to atmospheric pressure, so point D represents the normal boiling point of the solution.
So, Point D is the correct answer.
Note:
The difference between the Point D and Point C i.e., boiling point of the solution and boiling point of the solvent is known as the elevation in boiling point. It can be observed from the phase diagram that the boiling point of solution is higher than the boiling point of pure solvent.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




