
When a mixture of calcium benzoate and calcium acetate is dry distilled, the resulting compound is
A. Acetophenone
B. Benzaldehyde
C. Benzophenone
D. Acetaldehyde
Answer
579.6k+ views
Hint: Two molecules of different esters react to form a compound with ketone functional group thereby forming calcium carbonate as a by-product. In the first step dehydration occurs then in the other steps, the heating effect of calcium salts occurs.
In the above reaction, calcium benzoate is an ester with a benzene ring as a functional group and a calcium molecule. Similarly, calcium acetate is an ester with a straight chain of two carbon molecules and a calcium molecule.
Complete step by step solution:
Esters are formed as a result of reaction between hydrocarbons with alcohol functional groups and hydrocarbons with carboxylic acid functional groups. This type of reaction is called a condensation reaction as a water molecule is eliminated out of this reaction. This process of formation of ester is called the Esterification reaction.
This reaction is shown below
$C{H_3} - C{H_2} - OH + C{H_3} - C{H_2} - COOH \to C{H_3} - C{H_2} - COO - C{H_2} - C{H_3} + {H_2}O$
Similarly, in the above question the by-product formed is calcium carbonate $(CaCO_3)$ as a result of reaction between two esters. As already discussed calcium benzoate is an ester with a benzene ring as a functional group and a calcium molecule and calcium acetate is an ester with a straight chain of two carbon molecules and a calcium molecule. The reaction between the two involves elimination of the compound namely calcium carbonate $(CaCO_3)$ as a by-product and thus forms a compound with a ketone functional group. The reaction for the same is described below,
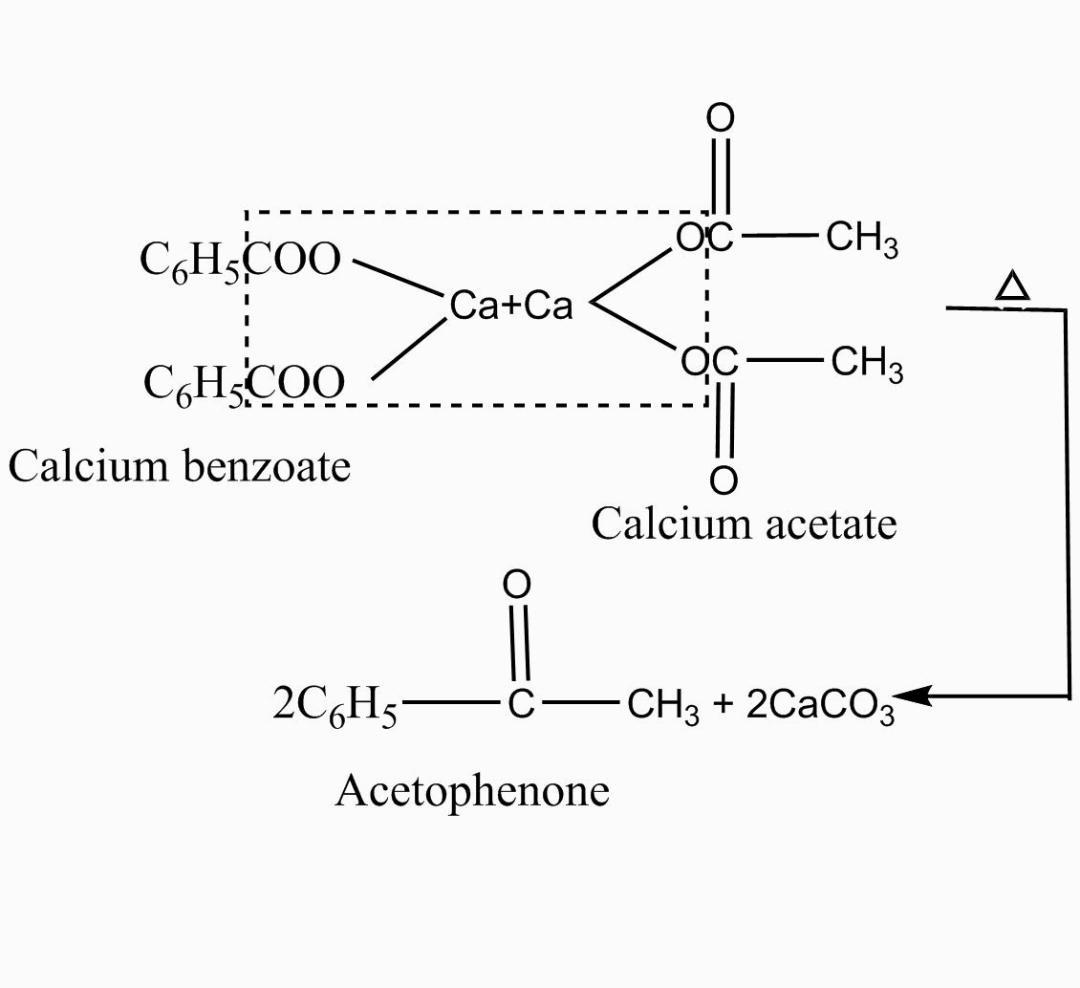
Hence, the resultant product formed as a result of dry distillation of the mixture of calcium benzoate and calcium acetate is a compound with a benzene ring, a ketone functional group with two carbon chains.
Thus, from the above mentioned options Acetophenone is a compound with formula $(C_6H_5 - CO - CH_3)$.
Hence, the correct option for the above question is option (A) Acetophenone
Note: Dry distillation is a process of conversion of solid materials or reactants to gaseous state with the application of heat. Hence, it is a heating process and no chemical change is involved but only the phase of the reactant changes.
In the above reaction, calcium benzoate is an ester with a benzene ring as a functional group and a calcium molecule. Similarly, calcium acetate is an ester with a straight chain of two carbon molecules and a calcium molecule.
Complete step by step solution:
Esters are formed as a result of reaction between hydrocarbons with alcohol functional groups and hydrocarbons with carboxylic acid functional groups. This type of reaction is called a condensation reaction as a water molecule is eliminated out of this reaction. This process of formation of ester is called the Esterification reaction.
This reaction is shown below
$C{H_3} - C{H_2} - OH + C{H_3} - C{H_2} - COOH \to C{H_3} - C{H_2} - COO - C{H_2} - C{H_3} + {H_2}O$
Similarly, in the above question the by-product formed is calcium carbonate $(CaCO_3)$ as a result of reaction between two esters. As already discussed calcium benzoate is an ester with a benzene ring as a functional group and a calcium molecule and calcium acetate is an ester with a straight chain of two carbon molecules and a calcium molecule. The reaction between the two involves elimination of the compound namely calcium carbonate $(CaCO_3)$ as a by-product and thus forms a compound with a ketone functional group. The reaction for the same is described below,

Hence, the resultant product formed as a result of dry distillation of the mixture of calcium benzoate and calcium acetate is a compound with a benzene ring, a ketone functional group with two carbon chains.
Thus, from the above mentioned options Acetophenone is a compound with formula $(C_6H_5 - CO - CH_3)$.
Hence, the correct option for the above question is option (A) Acetophenone
Note: Dry distillation is a process of conversion of solid materials or reactants to gaseous state with the application of heat. Hence, it is a heating process and no chemical change is involved but only the phase of the reactant changes.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




