
A magnesium ion has a net positive charge while the magnesium atom has no charge.
A.True
B.False
Answer
585.3k+ views
Hint: Magnesium belongs to group 2, alkaline earth metals. Every element placed in the periodic table is neutral means it has no charge when it is an atom. But every atom has electrons and vacant orbitals to gain or lose electrons and become ions from atoms.
Complete step by step answer:
-An atom comprises protons, neutrons and electrons. If an atom has an equal number of positively charged protons and negatively charged electrons, the atom is referred to as an electrically neutral atom.
-Atom has electrons revolving around the nucleus in their circular orbits. These orbits are made up of several orbitals in which electrons are placed as per their shells. When an electron is removed or lost from an atom, it becomes a positively charged ion. And if it gains or accepts an electron, it becomes a negatively charged ion.
-The gain or loss of an electron depends on the electronic configuration of an atom. Every atom tends to become stable by completing its octet and thus sharing electrons. Similarly, we can observe magnesium. When it is an atom, it has 12 protons and 12 electrons. Thus, it is a neutral atom.
-We know that electronic configuration of magnesium is \[1{s^2}2{s^2}2{p^6}3{s^2}\] . It has two electrons in 3s orbital and on losing these two electrons, its one shell will be removed and it will become more stable by coming close to the nucleus. Thus, whenever it loses these electrons, it becomes a positively charged ion. So, it is true that a magnesium ion has a net positive charge while the magnesium atom has no charge.
Hence, the correct option is (A).
Note:
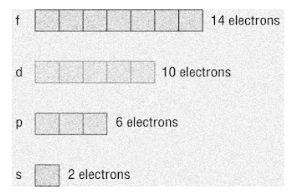
The orbitals in an atom are arranged as per the energy levels that are ordered as s, p, d and f. The higher the energy level, the more orbitals it has and a greater number of electrons it can accommodate. So, this means that s-orbital can accommodate two electrons, p-orbital can accommodate six electrons, ten electrons in d-orbital, fourteen electrons in f-orbitals and so on.
Complete step by step answer:
-An atom comprises protons, neutrons and electrons. If an atom has an equal number of positively charged protons and negatively charged electrons, the atom is referred to as an electrically neutral atom.
-Atom has electrons revolving around the nucleus in their circular orbits. These orbits are made up of several orbitals in which electrons are placed as per their shells. When an electron is removed or lost from an atom, it becomes a positively charged ion. And if it gains or accepts an electron, it becomes a negatively charged ion.
-The gain or loss of an electron depends on the electronic configuration of an atom. Every atom tends to become stable by completing its octet and thus sharing electrons. Similarly, we can observe magnesium. When it is an atom, it has 12 protons and 12 electrons. Thus, it is a neutral atom.
-We know that electronic configuration of magnesium is \[1{s^2}2{s^2}2{p^6}3{s^2}\] . It has two electrons in 3s orbital and on losing these two electrons, its one shell will be removed and it will become more stable by coming close to the nucleus. Thus, whenever it loses these electrons, it becomes a positively charged ion. So, it is true that a magnesium ion has a net positive charge while the magnesium atom has no charge.
Hence, the correct option is (A).
Note:
The orbitals in an atom are arranged as per the energy levels that are ordered as s, p, d and f. The higher the energy level, the more orbitals it has and a greater number of electrons it can accommodate. So, this means that s-orbital can accommodate two electrons, p-orbital can accommodate six electrons, ten electrons in d-orbital, fourteen electrons in f-orbitals and so on.

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




