
A Grignard reagent reacts with water to give:
(a)- Ether
(b)- Alkane
(c)- Amine
(d)- Alcohol
Answer
539.1k+ views
Hint: The Grignard reagent is a compound that comes under the organometallic compounds because there is carbon element as well as metal also present. The metal part is MgX, X is any halogen atom. In reaction with water, the MgX will react with the hydroxyl part of the water.
Complete answer:
When any compound reacts with water, we can say that a hydrolysis reaction occurs. The given compound in the question is the Grignard reagent.
We know that Grignard reagent is a compound that comes under the organometallic compounds because there is carbon element as well as metal also present. The general representation of the Grignard reagent is RMgX, where R is alkyl or aryl group and X is halogen like chlorine, bromine, or iodine.
In RMgX, the positive part of the cationic part is MgX, while the negative part or anionic part is the alkyl or aryl group. When the Grignard reagent reacts with water then the positive part of water, i.e., a hydrogen ion will react with the negative part of the Grignard reagent while the negative part of water, i.e., hydroxyl group will react with the positive part of the Grignard reagent. A general reaction is given below:
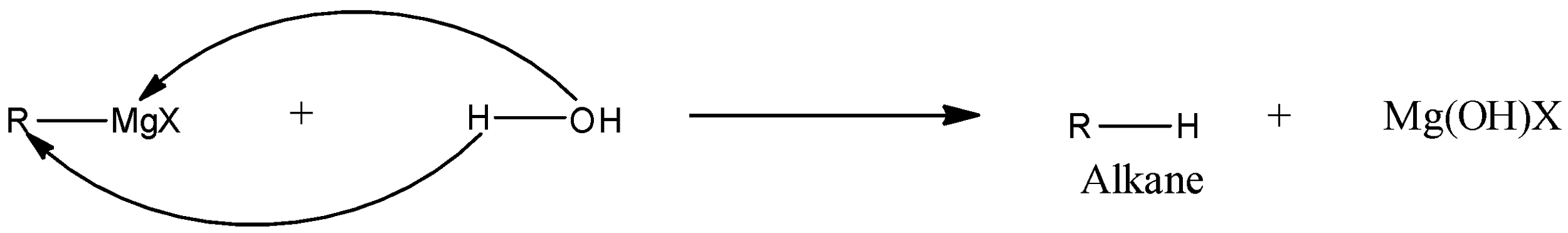
From the reaction, we can see that there is the formation of alkane.
Therefore, the correct answer is an option (b)- Alkane.
Note:
For example, you take $C{{H}_{3}}MgBr$ as the Grignard reagent and react it with water then it will form methane ($C{{H}_{4}}$). In the Grignard reagent, we cannot take fluorine as the halogen, only Cl, Br, or I can form Grignard reagent.
Complete answer:
When any compound reacts with water, we can say that a hydrolysis reaction occurs. The given compound in the question is the Grignard reagent.
We know that Grignard reagent is a compound that comes under the organometallic compounds because there is carbon element as well as metal also present. The general representation of the Grignard reagent is RMgX, where R is alkyl or aryl group and X is halogen like chlorine, bromine, or iodine.
In RMgX, the positive part of the cationic part is MgX, while the negative part or anionic part is the alkyl or aryl group. When the Grignard reagent reacts with water then the positive part of water, i.e., a hydrogen ion will react with the negative part of the Grignard reagent while the negative part of water, i.e., hydroxyl group will react with the positive part of the Grignard reagent. A general reaction is given below:
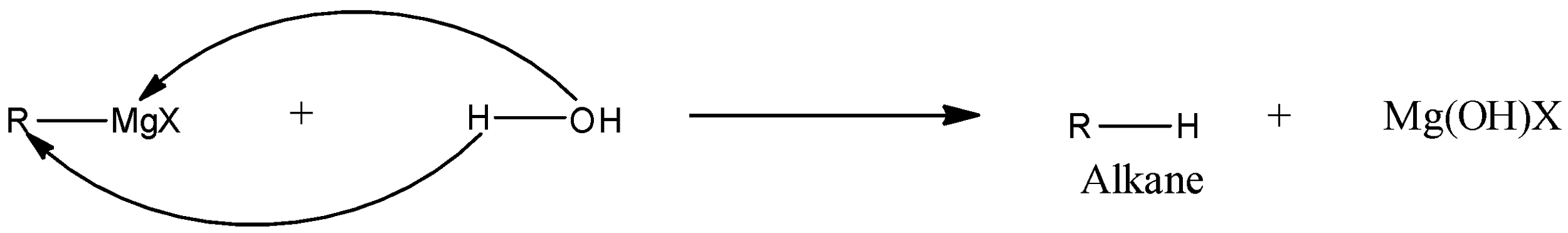
From the reaction, we can see that there is the formation of alkane.
Therefore, the correct answer is an option (b)- Alkane.
Note:
For example, you take $C{{H}_{3}}MgBr$ as the Grignard reagent and react it with water then it will form methane ($C{{H}_{4}}$). In the Grignard reagent, we cannot take fluorine as the halogen, only Cl, Br, or I can form Grignard reagent.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

State and prove Bernoullis theorem class 11 physics CBSE

Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE

Which out of the following hydrocarbons undergo addition class 11 chemistry CBSE




