
A flask contains a mixture of isohexane and ${\text{3 - methylpentane}}$ . one of the liquids boils at ${\text{6}}{{\text{0}}^{\text{0}}}{\text{c}}$ and the other one boils at
${\text{6}}{{\text{3}}^{\text{0}}}{\text{c}}$ . what is the best way to separate the two liquids and which one will be distilled out first?
A) fractional distillation, ${\text{3 - methylpentane}}$
B) simple distillation, isohexane
C)fractional distillation, isohexane
D) simple distillation, ${\text{3 - methylpentane}}$
Answer
580.2k+ views
Hint: Distillation is the process of separating components of a mixture based on different boiling points. It is used to separate a mixture of liquids, the liquid can be heated to force components, which have different boiling points, into the gas phase.
Complete Step by step answer: To separate two compounds having marginal difference in their boiling point, fractional distillation method is used
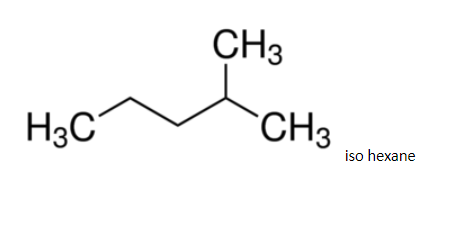
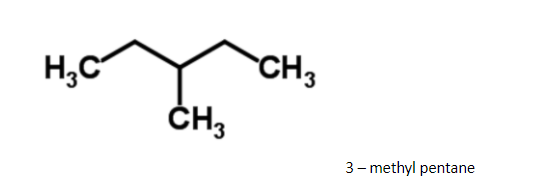
Since it has a regular structure, it will have a slightly higher boiling point.
Hence ${\text{3 - methylpentane}}$, it boils at ${\text{6}}{{\text{3}}^{\text{0}}}{\text{c}}$. The difference in boiling points is marginal, so, fractional distillation method is used. The compound having lower boiling point distils out first. So, isohexane distils out first and the method used for separation of such liquids is fractional distillation.
Generally the component parts have boiling points that differ by less than \[{{25^\circ C}}\] from each other under a pressure of one atmosphere. If the difference in boiling points is greater than \[{{25^\circ C}}\], a simple distillation is typically used.
Hence, option “C” is correct.
Note: Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize or boil.
Complete Step by step answer: To separate two compounds having marginal difference in their boiling point, fractional distillation method is used


Since it has a regular structure, it will have a slightly higher boiling point.
Hence ${\text{3 - methylpentane}}$, it boils at ${\text{6}}{{\text{3}}^{\text{0}}}{\text{c}}$. The difference in boiling points is marginal, so, fractional distillation method is used. The compound having lower boiling point distils out first. So, isohexane distils out first and the method used for separation of such liquids is fractional distillation.
Generally the component parts have boiling points that differ by less than \[{{25^\circ C}}\] from each other under a pressure of one atmosphere. If the difference in boiling points is greater than \[{{25^\circ C}}\], a simple distillation is typically used.
Hence, option “C” is correct.
Note: Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize or boil.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




