
A cyclic molecule that is a constitutional isomer of cyclohexane is:
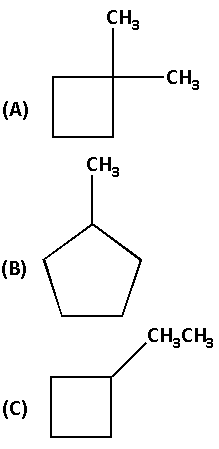
(D) All of the above

Answer
567.9k+ views
Hint:To solve this we must know that the molecules having the same molecular formula but different connectivity of atoms are known as constitutional isomers. We know that the molecular formula of cyclohexane is ${{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}$. We have to determine the molecular formula of each of the given molecules.
Complete answer:
We know that the molecules having the same molecular formula but different connectivity of atoms are known as constitutional isomers. If both the molecules have the same number of each atom and have different arrangements then they are said to be constitutional isomers of each other.
First let us draw the structure and molecular formula of cyclohexane.
The molecular formula of cyclohexane is ${{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}$. The structure of cyclohexane is as follows:
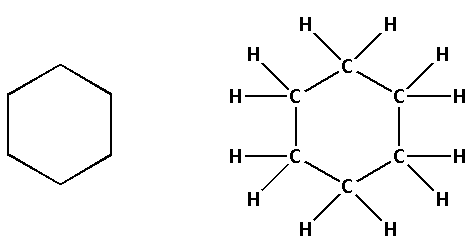
We are given three compounds.
The structure of compound (A) is as follows:
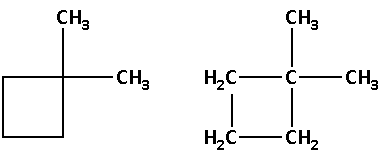
The molecular formula of compound (A) is ${{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}$.
Thus, the compound (A) is a constitutional isomer of cyclohexane.
The structure of compound (B) is as follows:
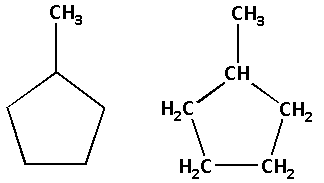
The molecular formula of compound (B) is ${{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}$.
Thus, the compound (B) is a constitutional isomer of cyclohexane.
The structure of compound (C) is as follows:
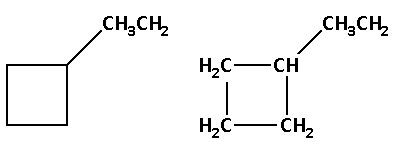
The molecular formula of compound (C) is ${{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}$.
Thus, the compound (C) is a constitutional isomer of cyclohexane.
Thus, the cyclic molecules that are constitutional isomers of cyclohexane are (A), (B) and (C).
Thus, the correct option is (D) all of the above.
Note:Remember that if both the molecules have the same number of each atom and have different arrangements then they are said to be constitutional isomers of each other. In simple words, the molecules that have the same molecular formula but different structures are said to be constitutional isomers of each other.
Complete answer:
We know that the molecules having the same molecular formula but different connectivity of atoms are known as constitutional isomers. If both the molecules have the same number of each atom and have different arrangements then they are said to be constitutional isomers of each other.
First let us draw the structure and molecular formula of cyclohexane.
The molecular formula of cyclohexane is ${{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}$. The structure of cyclohexane is as follows:

We are given three compounds.
The structure of compound (A) is as follows:

The molecular formula of compound (A) is ${{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}$.
Thus, the compound (A) is a constitutional isomer of cyclohexane.
The structure of compound (B) is as follows:

The molecular formula of compound (B) is ${{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}$.
Thus, the compound (B) is a constitutional isomer of cyclohexane.
The structure of compound (C) is as follows:

The molecular formula of compound (C) is ${{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}$.
Thus, the compound (C) is a constitutional isomer of cyclohexane.
Thus, the cyclic molecules that are constitutional isomers of cyclohexane are (A), (B) and (C).
Thus, the correct option is (D) all of the above.
Note:Remember that if both the molecules have the same number of each atom and have different arrangements then they are said to be constitutional isomers of each other. In simple words, the molecules that have the same molecular formula but different structures are said to be constitutional isomers of each other.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

State and prove Bernoullis theorem class 11 physics CBSE

Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE

The transition element that has lowest enthalpy of class 11 chemistry CBSE

Can anyone list 10 advantages and disadvantages of friction

State the laws of reflection of light




