
A cyclic molecule that is a constitutional isomer of cyclohexane is:
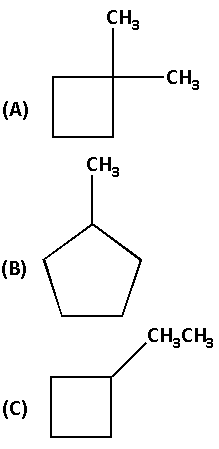
(D) All of the above

Answer
559.2k+ views
Hint:To solve this we must know that the molecules having the same molecular formula but different connectivity of atoms are known as constitutional isomers. We know that the molecular formula of cyclohexane is ${{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}$. We have to determine the molecular formula of each of the given molecules.
Complete answer:
We know that the molecules having the same molecular formula but different connectivity of atoms are known as constitutional isomers. If both the molecules have the same number of each atom and have different arrangements then they are said to be constitutional isomers of each other.
First let us draw the structure and molecular formula of cyclohexane.
The molecular formula of cyclohexane is ${{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}$. The structure of cyclohexane is as follows:
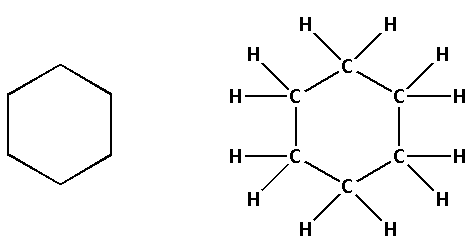
We are given three compounds.
The structure of compound (A) is as follows:
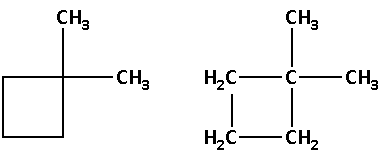
The molecular formula of compound (A) is ${{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}$.
Thus, the compound (A) is a constitutional isomer of cyclohexane.
The structure of compound (B) is as follows:
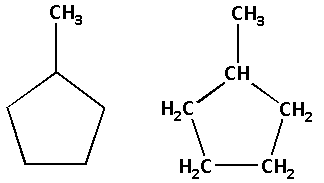
The molecular formula of compound (B) is ${{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}$.
Thus, the compound (B) is a constitutional isomer of cyclohexane.
The structure of compound (C) is as follows:
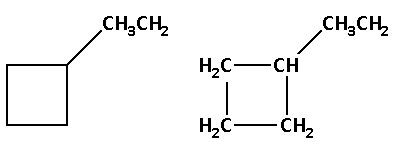
The molecular formula of compound (C) is ${{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}$.
Thus, the compound (C) is a constitutional isomer of cyclohexane.
Thus, the cyclic molecules that are constitutional isomers of cyclohexane are (A), (B) and (C).
Thus, the correct option is (D) all of the above.
Note:Remember that if both the molecules have the same number of each atom and have different arrangements then they are said to be constitutional isomers of each other. In simple words, the molecules that have the same molecular formula but different structures are said to be constitutional isomers of each other.
Complete answer:
We know that the molecules having the same molecular formula but different connectivity of atoms are known as constitutional isomers. If both the molecules have the same number of each atom and have different arrangements then they are said to be constitutional isomers of each other.
First let us draw the structure and molecular formula of cyclohexane.
The molecular formula of cyclohexane is ${{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}$. The structure of cyclohexane is as follows:

We are given three compounds.
The structure of compound (A) is as follows:

The molecular formula of compound (A) is ${{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}$.
Thus, the compound (A) is a constitutional isomer of cyclohexane.
The structure of compound (B) is as follows:

The molecular formula of compound (B) is ${{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}$.
Thus, the compound (B) is a constitutional isomer of cyclohexane.
The structure of compound (C) is as follows:

The molecular formula of compound (C) is ${{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}$.
Thus, the compound (C) is a constitutional isomer of cyclohexane.
Thus, the cyclic molecules that are constitutional isomers of cyclohexane are (A), (B) and (C).
Thus, the correct option is (D) all of the above.
Note:Remember that if both the molecules have the same number of each atom and have different arrangements then they are said to be constitutional isomers of each other. In simple words, the molecules that have the same molecular formula but different structures are said to be constitutional isomers of each other.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




