
A compound that gives a positive iodoform test is:
(A)- 1-pentanol
(B)- 2-pentanone
(C)- 3-pentanone
(D)- pentanal
Answer
593.4k+ views
Hint: The chemical formula of iodoform is $CHI_3$. An organic compound giving positive iodoform test leads to the formation of yellow precipitate of iodoform on reaction with basic (NaOH) solution and solid iodine.
Complete answer:
Iodoform test is generally used to detect the presence of methyl-ketone ($R-CO-C{{H}_{3}}$) . The alcohols which give iodoform test are of the type $C{{H}_{3}}-CHOH-R$. Here, R can be alkyl chain or hydrogen. Let us look at the structures of the compounds given above one by one:
1-propanol
Structure: \[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}OH\]
It does not contain ($C{{H}_{3}}-CH(OH)-$ ) group. So, it does not give iodoform test.
2-pentanone
Structure:
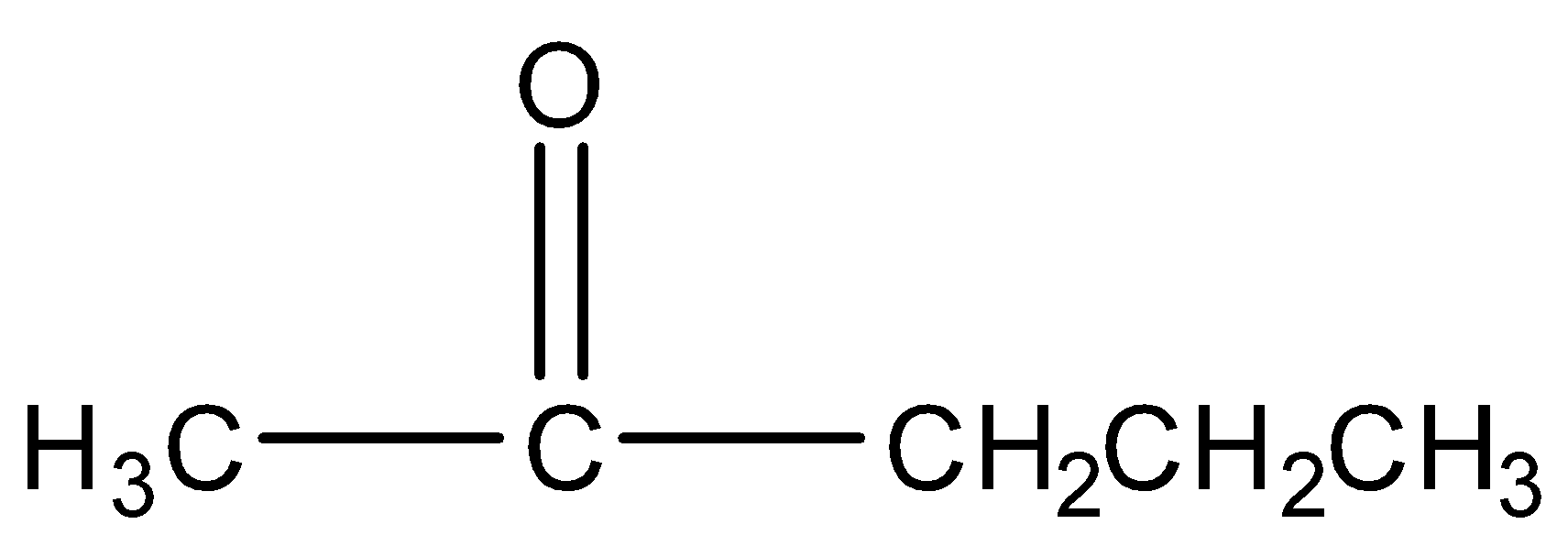
It contains an alpha methyl group. Therefore, gives a positive iodoform test.
3- pentanone
Structure:
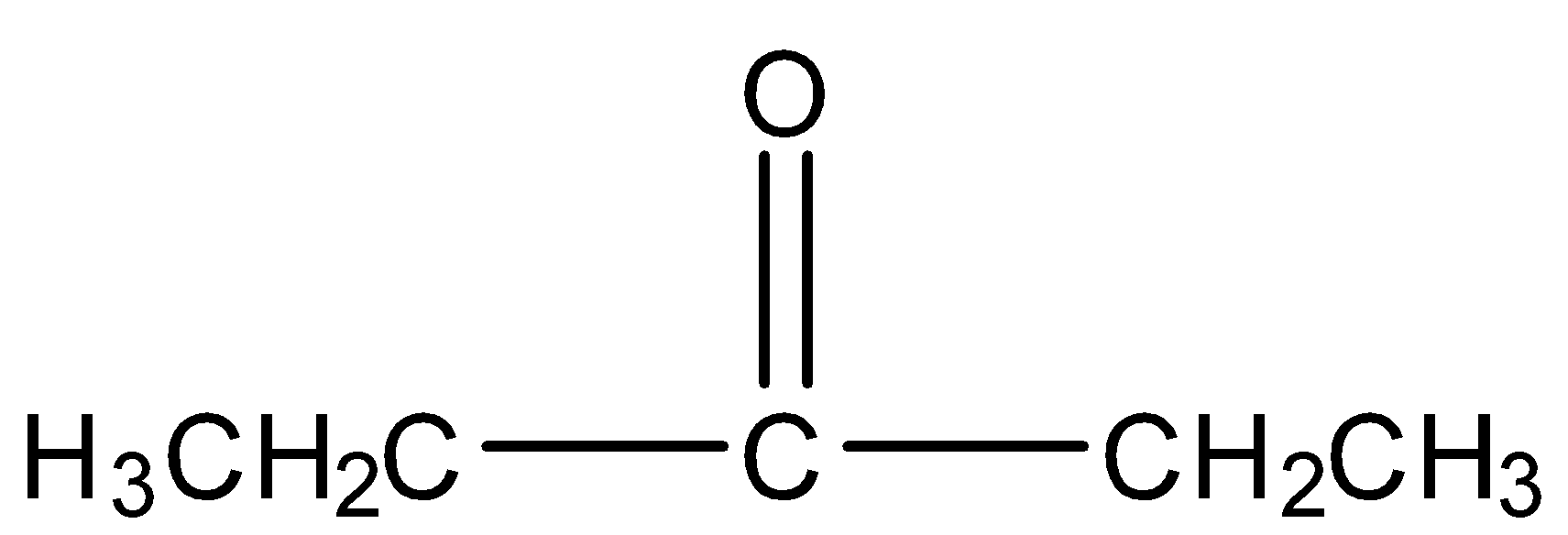
It is not a methyl ketone and does not give iodoform test.
Propanal
Structure:
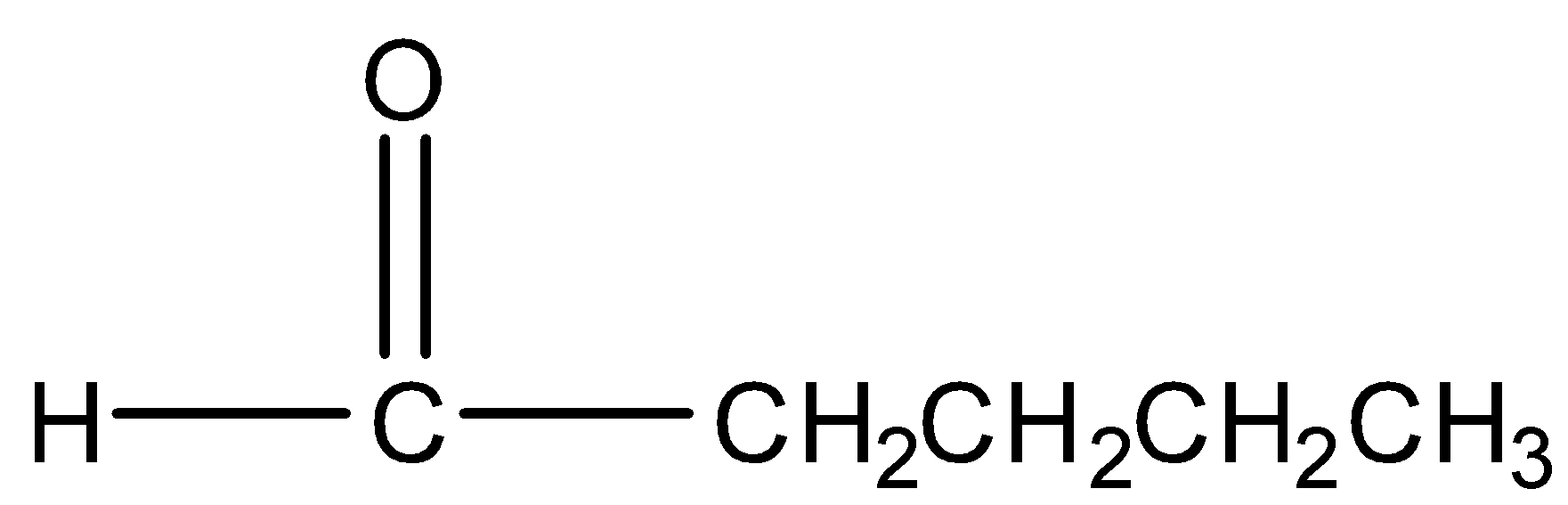
Generally aldehydes do not give iodoform test. Acetaldehyde (ethanal) is the only aldehyde that gives positive iodoform test because it contains \[C{{H}_{3}}CO-\] group. Thus, propanal does not give iodoform test.
Therefore, 2-pentanone shows positive iodoform test. The chemical reaction involved is given below:
So, the correct answer is “Option B”.
Additional Information:
Ethanol ($C{{H}_{3}}C{{H}_{2}}OH$) also gives iodoform test. Iodoform test is often used to distinguish ethanol and methanol.
Note: Note that we have to look for $C{{H}_{3}}CO-$ in carbonyl compounds and $C{{H}_{3}}CH(OH)-$ in alcohols to determine whether they give positive iodoform test or not. It is better to write the structure of the compounds to find out the presence of those groups in them.
Complete answer:
Iodoform test is generally used to detect the presence of methyl-ketone ($R-CO-C{{H}_{3}}$) . The alcohols which give iodoform test are of the type $C{{H}_{3}}-CHOH-R$. Here, R can be alkyl chain or hydrogen. Let us look at the structures of the compounds given above one by one:
1-propanol
Structure: \[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}OH\]
It does not contain ($C{{H}_{3}}-CH(OH)-$ ) group. So, it does not give iodoform test.
2-pentanone
Structure:

It contains an alpha methyl group. Therefore, gives a positive iodoform test.
3- pentanone
Structure:

It is not a methyl ketone and does not give iodoform test.
Propanal
Structure:

Generally aldehydes do not give iodoform test. Acetaldehyde (ethanal) is the only aldehyde that gives positive iodoform test because it contains \[C{{H}_{3}}CO-\] group. Thus, propanal does not give iodoform test.
Therefore, 2-pentanone shows positive iodoform test. The chemical reaction involved is given below:
\[C{{H}_{3}}COC{{H}_{2}}C{{H}_{2}}C{{H}_{3}}+4NaOH+3{{I}_{2}}\to CH{{I}_{3}}+C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}COONa+3NaI+3{{H}_{2}}O\]
So, the correct answer is “Option B”.
Additional Information:
Ethanol ($C{{H}_{3}}C{{H}_{2}}OH$) also gives iodoform test. Iodoform test is often used to distinguish ethanol and methanol.
Note: Note that we have to look for $C{{H}_{3}}CO-$ in carbonyl compounds and $C{{H}_{3}}CH(OH)-$ in alcohols to determine whether they give positive iodoform test or not. It is better to write the structure of the compounds to find out the presence of those groups in them.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




