
A common form of polyester is:
A.Nylon
B.Rayon
C.PET
D. Acrylic
Answer
590.1k+ views
Hint: We know that polymer is a huge compound formed from joining of repeating units (monomers) in a large scale. This process of polymer formation from monomers is coined as polymerization. Some examples of polymers are polyethene, nylon-6,6 etc.
Complete step by step answer:
Let’s first understand polyester in detail. Polyesters are polycondensation products of diols and dicarboxylic acid. Terylene or Dacron is one example of polyester. It can be produced by heating a mixture of terephthalic acid and ethylene glycol in presence of a catalyst like zinc acetate antimony trioxide.
Now, come to the question. We have to identify the common form of polyester. PET is polyethylene terephthalate (coon form of polyester). The chemical of its production is given below.
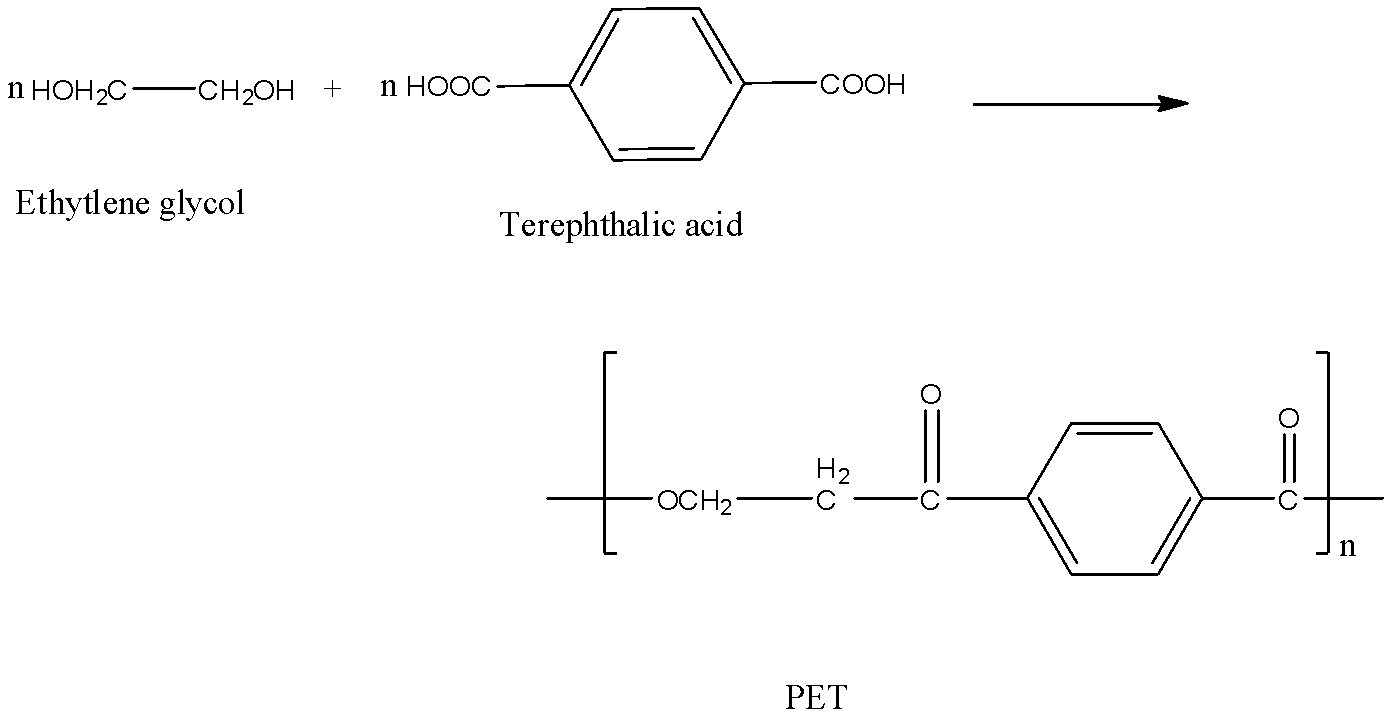
So, the correct answer is Option C.
Additional Information:
The polymers are classified on the basis on its source, its structure, its mode of polymerization etc. Now, we discuss the classification of polymer based on its source, that is, natural polymer, semisynthetic polymer and synthetic polymer.
Natural polymers are those polymers which are found in animals and plants, such as, protein, cellulose, rubber, starch, etc.
Semi synthetic polymers are the polymers mostly obtained by chemical modifications of naturally occurring polymers. Some examples are vulcanized rubber, rayon, etc.
Synthetic polymers are the polymers which are made in factories, such as, Buna-S, Buna-N etc.
Note:
PET is also known by the name Dacron. Dacron is the trademarked name of PET by an American company. PET is resistant to creases and therefore it is used in binding with wool and cotton fibres. It is also used as glass reinforcing materials in safety helmets.
Complete step by step answer:
Let’s first understand polyester in detail. Polyesters are polycondensation products of diols and dicarboxylic acid. Terylene or Dacron is one example of polyester. It can be produced by heating a mixture of terephthalic acid and ethylene glycol in presence of a catalyst like zinc acetate antimony trioxide.
Now, come to the question. We have to identify the common form of polyester. PET is polyethylene terephthalate (coon form of polyester). The chemical of its production is given below.

So, the correct answer is Option C.
Additional Information:
The polymers are classified on the basis on its source, its structure, its mode of polymerization etc. Now, we discuss the classification of polymer based on its source, that is, natural polymer, semisynthetic polymer and synthetic polymer.
Natural polymers are those polymers which are found in animals and plants, such as, protein, cellulose, rubber, starch, etc.
Semi synthetic polymers are the polymers mostly obtained by chemical modifications of naturally occurring polymers. Some examples are vulcanized rubber, rayon, etc.
Synthetic polymers are the polymers which are made in factories, such as, Buna-S, Buna-N etc.
Note:
PET is also known by the name Dacron. Dacron is the trademarked name of PET by an American company. PET is resistant to creases and therefore it is used in binding with wool and cotton fibres. It is also used as glass reinforcing materials in safety helmets.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




