
What is a bomb calorimeter explaining the working of a bomb calorimeter and its construction?
Answer
547.5k+ views
Hint:It is used to determine energy change which occurs during a reaction accurately. The modern one is a development of the original calorimeter of Berthelot. It is made of steel capable of resisting the process and deprivation which might occur if corrosion happens. This calorimeter is an instrument that is used to measure heat of reaction at fixed volume and the heat which is measured is called the change of internal energy $(\Delta E)$.
Complete step-by-step answer:When we talk about chemistry, we must know that changes in heat of a reaction can be measured at fixed pressure and volume. When we talk about its working, we must know that it is a type of constant-volume calorimeter which can be used to measure the combustion heat of samples which can be burnt in oxygen. When we talk about the critical part, there are four of them. It is a laboratory instrument which is used to measure the amount of heat power too. The purpose of this is to determine the effect of using bomb calorimeter to process how science work.
For hydrocarbons, it is used to determine the enthalpy of combustion, \[{\Delta _{comb}}H\;\]which is found by:
\[{C_x}{H_Y}{O_{z{\text{ }}(s)}}\;\; + \;\;\left( {2X + Y/2 - Z} \right)/2\;{O_{2{\text{ }}(g)}}\;\; \to \;\;X\;C{O_{2{\text{ }}(g)}}\; + \;Y\;{H_2}{O_{\;(l)}}\]
It gives out heat and thus an exothermic reaction which means that \[{\Delta _{comb}}H\;\]is negative.
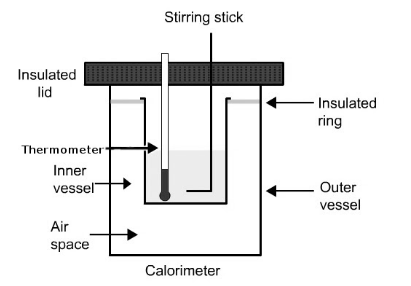
When we talk about its construction we must know that it consists of a sample, oxygen, stainless stell, bomb and water. There is a dewar which prevents heat flow to the surrounding. This thus makes the following situation possible:
\[{q_{calorimeter}}\; = {\text{ }}0\]
Note: The bomb is made up of stainless steel. This makes combustion rection to occur at constant volume possible. This means no work is done. The equation for the same is:
\[{w_{calorimeter}}\; = \; - \smallint \;p\;dV\; = {\text{ }}0\]
Thus, the change in internal energy, DU, for the calorimeter is zero
\[{U_{calorimeter}}\; = \;{q_{calorimeter}}\; + \;{w_{calorimeter}}\; = {\text{ }}0\]
The thermodynamic interpretation of this equation is that the calorimeter is isolated from the rest of the universe.
Complete step-by-step answer:When we talk about chemistry, we must know that changes in heat of a reaction can be measured at fixed pressure and volume. When we talk about its working, we must know that it is a type of constant-volume calorimeter which can be used to measure the combustion heat of samples which can be burnt in oxygen. When we talk about the critical part, there are four of them. It is a laboratory instrument which is used to measure the amount of heat power too. The purpose of this is to determine the effect of using bomb calorimeter to process how science work.
For hydrocarbons, it is used to determine the enthalpy of combustion, \[{\Delta _{comb}}H\;\]which is found by:
\[{C_x}{H_Y}{O_{z{\text{ }}(s)}}\;\; + \;\;\left( {2X + Y/2 - Z} \right)/2\;{O_{2{\text{ }}(g)}}\;\; \to \;\;X\;C{O_{2{\text{ }}(g)}}\; + \;Y\;{H_2}{O_{\;(l)}}\]
It gives out heat and thus an exothermic reaction which means that \[{\Delta _{comb}}H\;\]is negative.
When we talk about its construction we must know that it consists of a sample, oxygen, stainless stell, bomb and water. There is a dewar which prevents heat flow to the surrounding. This thus makes the following situation possible:
\[{q_{calorimeter}}\; = {\text{ }}0\]

Note: The bomb is made up of stainless steel. This makes combustion rection to occur at constant volume possible. This means no work is done. The equation for the same is:
\[{w_{calorimeter}}\; = \; - \smallint \;p\;dV\; = {\text{ }}0\]
Thus, the change in internal energy, DU, for the calorimeter is zero
\[{U_{calorimeter}}\; = \;{q_{calorimeter}}\; + \;{w_{calorimeter}}\; = {\text{ }}0\]
The thermodynamic interpretation of this equation is that the calorimeter is isolated from the rest of the universe.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




