
5 moles of \[Ba{{(OH)}_{2}}\]are treated with excess of\[C{{O}_{2}}\] . How much \[BaC{{O}_{3}}\]will be formed?
(a) 39.4g
(b) 197g
(c) 591g
(d) 985g
Answer
597.3k+ views
Hint: First the chemical equation is written in the balanced form. For finding the mass of element formed molecular mass the compound be calculated. The number of moles should be multiplied with molar mass.
Complete answer:
Let us understand the Stoichiometry of a chemical reaction.
It is the major aspect of a chemical equation. In the balanced form, it gives a quantitative relationship between the various reactants and products in terms of moles, masses, molecules, and volumes. Here is an example of a balanced equation with quantitative information:
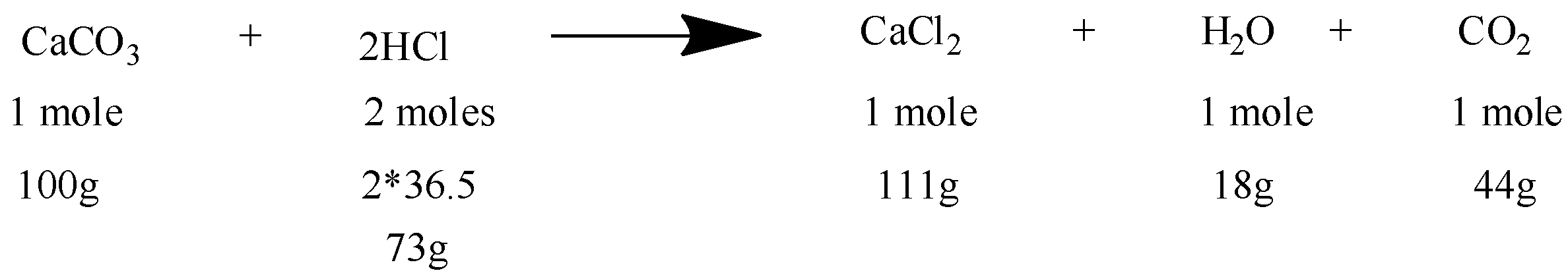
Hence, there is a general method used for the calculation:
Write down the balanced chemical equation.
Write the relative number of moles or relative mass of the reactant and the product below their formulae.
In the case of the gaseous substance, write 22.4 liters at STP below the formula.
Apply unitary method to make required calculations.
So, by following all these steps, we can solve the question.
5 moles of\[Ba{{(OH)}_{2}}\] react with excess of \[C{{O}_{2}}\] to form \[BaC{{O}_{3}}\]
The balanced reaction will be:

5 moles\[Ba{{(OH)}_{2}}\] react with excess of \[C{{O}_{2}}\]will form 5 moles of \[BaC{{O}_{3}}\]
So,

The molecular mass of\[Ba{{(OH)}_{2}}\] is 171.34 g/mol
The mass of\[Ba{{(OH)}_{2}}\] reacted = 5 * 171.34 = 856.7g
The molecular mass of \[BaC{{O}_{3}}\] is 197g/mol
Then, the mass of \[BaC{{O}_{3}}\] formed after the reaction will be = 5 * 197 = 985g
Hence, the mass formed is 985g.
A correct answer is an option (d) 985g.
Note: Make sure that whenever you are solving these types of questions, balance the equation correctly otherwise it will have the wrong result. You may also get confused with option (b) because in the balanced equation there is only one mole of barium carbonate. Hence, the answer would come 197g but the number of moles should also be taken care of.
Complete answer:
Let us understand the Stoichiometry of a chemical reaction.
It is the major aspect of a chemical equation. In the balanced form, it gives a quantitative relationship between the various reactants and products in terms of moles, masses, molecules, and volumes. Here is an example of a balanced equation with quantitative information:

Hence, there is a general method used for the calculation:
Write down the balanced chemical equation.
Write the relative number of moles or relative mass of the reactant and the product below their formulae.
In the case of the gaseous substance, write 22.4 liters at STP below the formula.
Apply unitary method to make required calculations.
So, by following all these steps, we can solve the question.
5 moles of\[Ba{{(OH)}_{2}}\] react with excess of \[C{{O}_{2}}\] to form \[BaC{{O}_{3}}\]
The balanced reaction will be:

5 moles\[Ba{{(OH)}_{2}}\] react with excess of \[C{{O}_{2}}\]will form 5 moles of \[BaC{{O}_{3}}\]
So,

The molecular mass of\[Ba{{(OH)}_{2}}\] is 171.34 g/mol
The mass of\[Ba{{(OH)}_{2}}\] reacted = 5 * 171.34 = 856.7g
The molecular mass of \[BaC{{O}_{3}}\] is 197g/mol
Then, the mass of \[BaC{{O}_{3}}\] formed after the reaction will be = 5 * 197 = 985g
Hence, the mass formed is 985g.
A correct answer is an option (d) 985g.
Note: Make sure that whenever you are solving these types of questions, balance the equation correctly otherwise it will have the wrong result. You may also get confused with option (b) because in the balanced equation there is only one mole of barium carbonate. Hence, the answer would come 197g but the number of moles should also be taken care of.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

State and prove converse of BPT Basic Proportionality class 10 maths CBSE




