
$448{\text{ ml}}$ of a gaseous hydrocarbon (A) having \[{\text{C }}87.80\% \], \[{\text{H }}12.19\% \] weighs $1.64{\text{ gms}}$ at STP. On hydrogenation it gives 2-methyl pentane. Treatment of (A) with acidic ${\text{HgS}}{{\text{O}}_{\text{4}}}$ gives a new compound (B) M.F. ${{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}{\text{O}}$. Compound (A) does not react with ammoniacal ${\text{AgN}}{{\text{O}}_{\text{3}}}$. Find out the structure of (A) and explain the reactions involved.
Answer
561.6k+ views
Hint: To solve this first calculate the molecular mass of the hydrocarbon. Then determine the molecular formula of the hydrocarbon from its empirical formula. We are given that hydrocarbon on hydrogenation gives an alkane. Thus, the hydrocarbon can be an alkene or alkyne.
Complete step-by-step solution:
We know that one mole of an ideal gas occupies a volume of $22.4{\text{ L}} = {\text{22}}{\text{.4}} \times {\text{1}}{{\text{0}}^3}{\text{ ml}}$ at STP i.e. standard temperature and pressure.
We are given that $448{\text{ ml}}$ of a gaseous hydrocarbon (A) weighs $1.64{\text{ gms}}$ at STP. Thus, ${\text{22}}{\text{.4}} \times {\text{1}}{{\text{0}}^3}{\text{ ml}}$ of gas at STP will weigh,
${\text{Weight of hydrocarbon}} = 1.64{\text{ g}} \times \dfrac{{22.4 \times {{10}^3}{\text{ ml}}}}{{448{\text{ ml}}}} = 82{\text{ g}}$
Thus, one mole of hydrocarbon weighs $82{\text{ g}}$ at STP. Thus, the molecular weight of the hydrocarbon (A) is $82{\text{ g}}$.
Determine the empirical formula of the hydrocarbon (A) as follows:
We are given that hydrocarbon (A) has \[{\text{C }}87.80\% \], \[{\text{H }}12.19\% \]. Thus,
We multiply the relative number of atoms by 3 to get a simplest whole number ratio.
Thus, the empirical formula of the hydrocarbon (A) is ${{\text{C}}_{\text{3}}}{{\text{H}}_{\text{5}}}$.
Determine the empirical formula mass as follows:
${\text{Empirical formula mass}} = \left( {3 \times {\text{C}}} \right) + \left( {5 \times {\text{H}}} \right)$
${\text{Empirical formula mass}} = \left( {3 \times 12} \right) + \left( {5 \times 1} \right)$
${\text{Empirical formula mass}} = 41$
Thus, the empirical formula mass of the hydrocarbon (A) is 41.
Determine the molecular formula of the hydrocarbon (A) as follows:
$n = \dfrac{{{\text{Molecular mass}}}}{{{\text{Empirical formula mass}}}}$
$n = \dfrac{{{\text{82}}}}{{{\text{41}}}} = 2$
Thus,
${\text{Molecular formula}} = {\text{Empirical formula}} \times n$
${\text{Molecular formula}} = {{\text{C}}_3}{{\text{H}}_5} \times 2$
${\text{Molecular formula}} = {{\text{C}}_6}{{\text{H}}_{10}} \times 2$
Thus, the molecular formula of the hydrocarbon (A) is ${{\text{C}}_6}{{\text{H}}_{10}}$.
We are given that hydrocarbon (A) does not react with ammoniacal ${\text{AgN}}{{\text{O}}_{\text{3}}}$. Internal alkynes do not react with ammoniacal ${\text{AgN}}{{\text{O}}_{\text{3}}}$. Thus, hydrocarbon (A) is an internal alkyne
We are given that hydrocarbon (A) on hydrogenation gives 2-methyl pentane which is an alkane. Alkynes on reaction with 2 moles of hydrogen gas give alkane. Thus, hydrocarbon (A) must be an internal alkyne with five carbon chains and methyl substituent at carbon number 4.
Thus, the structure of hydrocarbon (A) is as follows:
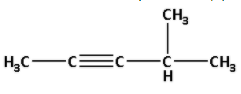
Thus, hydrocarbon (A) is 4-methyl pent-2-yne.
The reaction of hydrocarbon (A) with 2 moles of hydrogen gas is as follows:
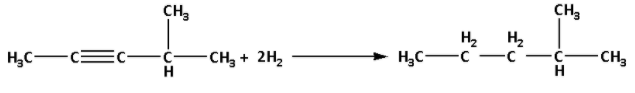
We are given that hydrocarbon (A) reacts with acidic ${\text{HgS}}{{\text{O}}_{\text{4}}}$ and gives a new compound (B) having M.F. ${{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}{\text{O}}$.
Reaction of an alkyne with acidic ${\text{HgS}}{{\text{O}}_{\text{4}}}$ gives an aldehyde or ketone. Thus, compound B can be an aldehyde or a ketone.
Note: Remember that terminal alkynes do not react with ammoniacal silver nitrate. Only internal alkynes react with ammoniacal silver nitrate. Terminal alkynes are alkynes having a triple bond at terminal position whereas internal alkynes are non-terminal alkynes.
Complete step-by-step solution:
We know that one mole of an ideal gas occupies a volume of $22.4{\text{ L}} = {\text{22}}{\text{.4}} \times {\text{1}}{{\text{0}}^3}{\text{ ml}}$ at STP i.e. standard temperature and pressure.
We are given that $448{\text{ ml}}$ of a gaseous hydrocarbon (A) weighs $1.64{\text{ gms}}$ at STP. Thus, ${\text{22}}{\text{.4}} \times {\text{1}}{{\text{0}}^3}{\text{ ml}}$ of gas at STP will weigh,
${\text{Weight of hydrocarbon}} = 1.64{\text{ g}} \times \dfrac{{22.4 \times {{10}^3}{\text{ ml}}}}{{448{\text{ ml}}}} = 82{\text{ g}}$
Thus, one mole of hydrocarbon weighs $82{\text{ g}}$ at STP. Thus, the molecular weight of the hydrocarbon (A) is $82{\text{ g}}$.
Determine the empirical formula of the hydrocarbon (A) as follows:
We are given that hydrocarbon (A) has \[{\text{C }}87.80\% \], \[{\text{H }}12.19\% \]. Thus,
| Element(Given) | Percentage(Given) | Atomic mass(Known) | Relative ratio(Percentage/Atomic mass) | Relative number of atoms(Relative ratio/ Smallest relative ratio) | Simplest ratioRelative number of atoms multiplied by 3 |
| ${\text{C}}$ | 87.80 | 12 | 7.31 | 1 | 3 |
| ${\text{H}}$ | 12.19 | 1 | 12.19 | 1.66 | 5 |
We multiply the relative number of atoms by 3 to get a simplest whole number ratio.
Thus, the empirical formula of the hydrocarbon (A) is ${{\text{C}}_{\text{3}}}{{\text{H}}_{\text{5}}}$.
Determine the empirical formula mass as follows:
${\text{Empirical formula mass}} = \left( {3 \times {\text{C}}} \right) + \left( {5 \times {\text{H}}} \right)$
${\text{Empirical formula mass}} = \left( {3 \times 12} \right) + \left( {5 \times 1} \right)$
${\text{Empirical formula mass}} = 41$
Thus, the empirical formula mass of the hydrocarbon (A) is 41.
Determine the molecular formula of the hydrocarbon (A) as follows:
$n = \dfrac{{{\text{Molecular mass}}}}{{{\text{Empirical formula mass}}}}$
$n = \dfrac{{{\text{82}}}}{{{\text{41}}}} = 2$
Thus,
${\text{Molecular formula}} = {\text{Empirical formula}} \times n$
${\text{Molecular formula}} = {{\text{C}}_3}{{\text{H}}_5} \times 2$
${\text{Molecular formula}} = {{\text{C}}_6}{{\text{H}}_{10}} \times 2$
Thus, the molecular formula of the hydrocarbon (A) is ${{\text{C}}_6}{{\text{H}}_{10}}$.
We are given that hydrocarbon (A) does not react with ammoniacal ${\text{AgN}}{{\text{O}}_{\text{3}}}$. Internal alkynes do not react with ammoniacal ${\text{AgN}}{{\text{O}}_{\text{3}}}$. Thus, hydrocarbon (A) is an internal alkyne
We are given that hydrocarbon (A) on hydrogenation gives 2-methyl pentane which is an alkane. Alkynes on reaction with 2 moles of hydrogen gas give alkane. Thus, hydrocarbon (A) must be an internal alkyne with five carbon chains and methyl substituent at carbon number 4.
Thus, the structure of hydrocarbon (A) is as follows:

Thus, hydrocarbon (A) is 4-methyl pent-2-yne.
The reaction of hydrocarbon (A) with 2 moles of hydrogen gas is as follows:
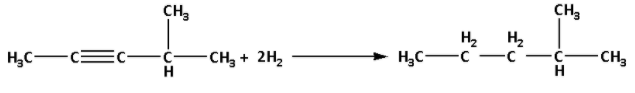
We are given that hydrocarbon (A) reacts with acidic ${\text{HgS}}{{\text{O}}_{\text{4}}}$ and gives a new compound (B) having M.F. ${{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}{\text{O}}$.
Reaction of an alkyne with acidic ${\text{HgS}}{{\text{O}}_{\text{4}}}$ gives an aldehyde or ketone. Thus, compound B can be an aldehyde or a ketone.
Note: Remember that terminal alkynes do not react with ammoniacal silver nitrate. Only internal alkynes react with ammoniacal silver nitrate. Terminal alkynes are alkynes having a triple bond at terminal position whereas internal alkynes are non-terminal alkynes.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




