
When 1-iodo-1-methylcyclohexane is treated with $ NaOC{H_2}C{H_3} $ as the base, the more highly substituted alkene product predominates. When $ KOC{(C{H_3})_3} $ is used as the base, then less substituted alkene predominates. Why? What are the structures of the two products?
Answer
504k+ views
Hint: Steric effects are the nonbonding interactions which influence the conformation and reactivity of the ions and molecules in a chemical reaction. The products in the elimination reaction are influenced by the steric effect of the base considered in the reaction and form products accordingly.
Complete answer:
The product in the given reaction conditions follows Saytzeff’s rule and Hoffman rule. Saytzeff’s rule states that in an elimination reaction, the more substituted alkene is most favoured. But, steric interaction within the substrate prevents the formation of Saytzeff product and in that case, the formation of least substituted alkene takes place which is referred to as the Hoffman rule.
Now, as per question, the two bases considered in the reaction are $ NaOC{H_2}C{H_3} $ and $ KOC{(C{H_3})_3} $ . The ethoxide ion i.e., $ C{H_3}C{H_2}{O^ - } $ is small and sterically unhindered while the tertiary butoxide ion i.e., $ (C{H_3})C{O^ - } $ is bulky and sterically hindered.
Thus, in case of $ NaOC{H_2}C{H_3} $ , the product is formed as per Saytzeff’s rule and the reaction takes place as follows:
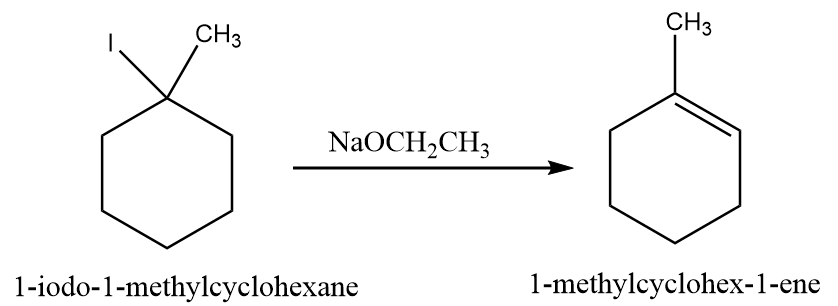
While in case of $ KOC{(C{H_3})_3} $ , the product is formed as per Hoffman’s rule and the reaction takes place as follows:
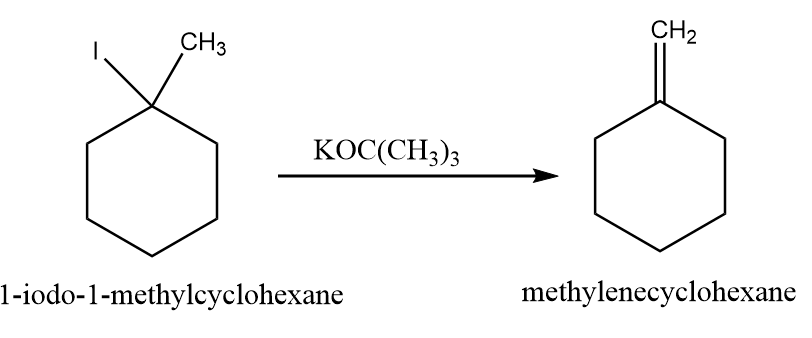
Hence, due to steric hindrance, $ NaOC{H_2}C{H_3} $ favours more substituted alkene whereas when $ KOC{(C{H_3})_3} $ is taken as base, then less substituted alkene predominates and the products formed in both the case are 1-methylcyclohex-1-ene and methylenecyclohexane respectively.
Note:
It is important to note that the elimination reactions are always regioselective, that means the products formed in the reaction are constitutional isomers to each other. During the reaction, the more stable product (either Saytzeff or Hoffman as per reaction conditions) is major while its less stable constitutional isomer is considered as a minor product.
Complete answer:
The product in the given reaction conditions follows Saytzeff’s rule and Hoffman rule. Saytzeff’s rule states that in an elimination reaction, the more substituted alkene is most favoured. But, steric interaction within the substrate prevents the formation of Saytzeff product and in that case, the formation of least substituted alkene takes place which is referred to as the Hoffman rule.
Now, as per question, the two bases considered in the reaction are $ NaOC{H_2}C{H_3} $ and $ KOC{(C{H_3})_3} $ . The ethoxide ion i.e., $ C{H_3}C{H_2}{O^ - } $ is small and sterically unhindered while the tertiary butoxide ion i.e., $ (C{H_3})C{O^ - } $ is bulky and sterically hindered.
Thus, in case of $ NaOC{H_2}C{H_3} $ , the product is formed as per Saytzeff’s rule and the reaction takes place as follows:

While in case of $ KOC{(C{H_3})_3} $ , the product is formed as per Hoffman’s rule and the reaction takes place as follows:

Hence, due to steric hindrance, $ NaOC{H_2}C{H_3} $ favours more substituted alkene whereas when $ KOC{(C{H_3})_3} $ is taken as base, then less substituted alkene predominates and the products formed in both the case are 1-methylcyclohex-1-ene and methylenecyclohexane respectively.
Note:
It is important to note that the elimination reactions are always regioselective, that means the products formed in the reaction are constitutional isomers to each other. During the reaction, the more stable product (either Saytzeff or Hoffman as per reaction conditions) is major while its less stable constitutional isomer is considered as a minor product.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




