
1. $\Delta U$ is a state function.
2. Heat and work are not state functions.
(A)- only 1 is true
(B)- only 2 is true
(C)- both are true
(D)- none of the above
Answer
594.6k+ views
Hint: If a property or quantity depends on the state of the system (initial and final), it is called as state function whereas if it depends on the path followed to attain that state then it is called as path function.
Complete step by step solution:
Let us consider the two statements given one by one:
Internal energy of a system is a state function. Its value depends only on the initial and final states of the system i.e. its temperature, pressure, volume, etc. It is independent of the path that has been followed to reach that state. To explain that the internal energy is a state function let us consider an example.
Consider one mole of carbon dioxide gas and its temperature is reduced from 500 K to 300 K and pressure is lowered from 5 atmospheric to 1 atmospheric pressure.
Consider the same system at 1000 K and 10 atmospheric pressure. Now the temperature is reduced from 1000 K to 300 K and pressure is reduced to 1 atmospheric from 10 atmospheric pressure.
In both the cases the internal energy of the carbon dioxide at 300 K temperature and 1 atmospheric pressure will be the same. It is not important whether the gas has been brought to this state of 300 K temperature and 1 atmospheric pressure from 1000 K and 10 atmospheric or 500 K and 5 atmospheric pressure.
Therefore, statement 1 is true.
Heat and work are not state functions. Their values do not depend on the initial and final states but on the path followed to attain the state. Let us consider an example to prove it.
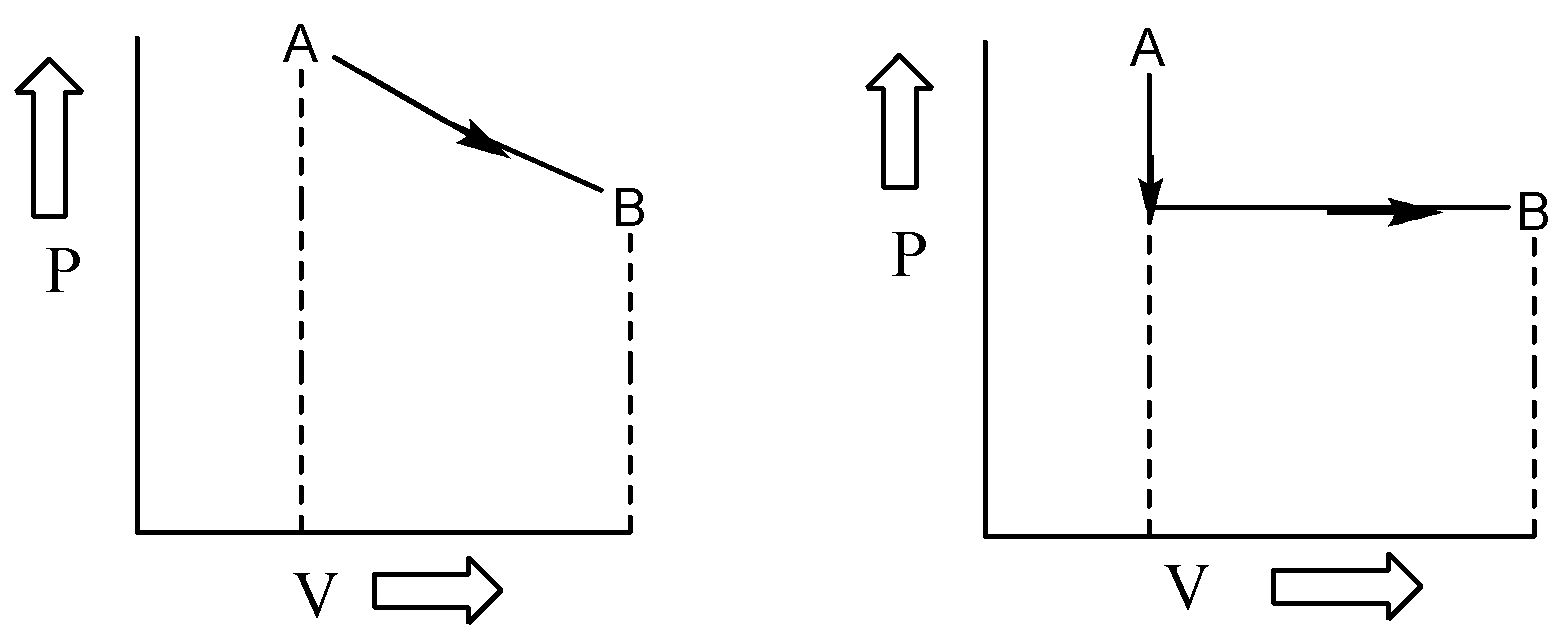
Consider a pressure-volume plot for a system undergoing a change from point A to B. work done by the system to change volume from V to V+dV will be given by P dV. The total work done will be the summation of P dV at every step which can be obtained by integrating all the small work done terms. The total work done is equal to the area under the curve. Now for both the cases, the initial and final states are the same but the area under the curve is different i.e. work done in both the cases is different. Hence, work is not a state function but a path function.
Similarly, the heat or energy change involved in the processes will be different. Also, from the first law of thermodynamics, we have
\[\begin{align}
& \Delta U=q+w \\
& q=\Delta U-w \\
\end{align}\]
Difference of a state function ($\Delta U$) and a path function (w) gives a path function. Thus, both heat and work are not state functions.
Therefore, statement 2 is also true.
So, the correct answer is “Option C”.
Note: It is to be noted here that heat is also a form of energy but it is not a state function as internal energy because the amount of heat evolved or absorbed depends on the type of work done. For example, in adiabatic work, heat (q) is zero.
Complete step by step solution:
Let us consider the two statements given one by one:
Internal energy of a system is a state function. Its value depends only on the initial and final states of the system i.e. its temperature, pressure, volume, etc. It is independent of the path that has been followed to reach that state. To explain that the internal energy is a state function let us consider an example.
Consider one mole of carbon dioxide gas and its temperature is reduced from 500 K to 300 K and pressure is lowered from 5 atmospheric to 1 atmospheric pressure.
Consider the same system at 1000 K and 10 atmospheric pressure. Now the temperature is reduced from 1000 K to 300 K and pressure is reduced to 1 atmospheric from 10 atmospheric pressure.
In both the cases the internal energy of the carbon dioxide at 300 K temperature and 1 atmospheric pressure will be the same. It is not important whether the gas has been brought to this state of 300 K temperature and 1 atmospheric pressure from 1000 K and 10 atmospheric or 500 K and 5 atmospheric pressure.
Therefore, statement 1 is true.
Heat and work are not state functions. Their values do not depend on the initial and final states but on the path followed to attain the state. Let us consider an example to prove it.
Consider a pressure-volume plot for a system undergoing a change from point A to B. work done by the system to change volume from V to V+dV will be given by P dV. The total work done will be the summation of P dV at every step which can be obtained by integrating all the small work done terms. The total work done is equal to the area under the curve. Now for both the cases, the initial and final states are the same but the area under the curve is different i.e. work done in both the cases is different. Hence, work is not a state function but a path function.

Similarly, the heat or energy change involved in the processes will be different. Also, from the first law of thermodynamics, we have
\[\begin{align}
& \Delta U=q+w \\
& q=\Delta U-w \\
\end{align}\]
Difference of a state function ($\Delta U$) and a path function (w) gives a path function. Thus, both heat and work are not state functions.
Therefore, statement 2 is also true.
So, the correct answer is “Option C”.
Note: It is to be noted here that heat is also a form of energy but it is not a state function as internal energy because the amount of heat evolved or absorbed depends on the type of work done. For example, in adiabatic work, heat (q) is zero.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




