Answer
37.2k+ views
Hint:Potassium persulfate is an inorganic compound having a formula of ${ K }_{ 2 }{ S }_{ 2 }{ O }_{ 8 }$. It is also known as potassium peroxydisulfate. Chromyl chloride is a reddish-brown compound having a formula of ${ CrO }_{ 2 }{ Cl }_{ 2 }$.
Complete step by step answer:
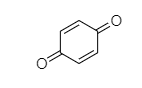
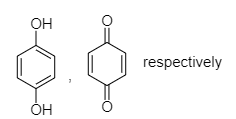
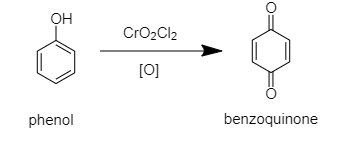
When phenol reacts with chromyl chloride in presence of nascent oxygen then benzoquinone will be formed.
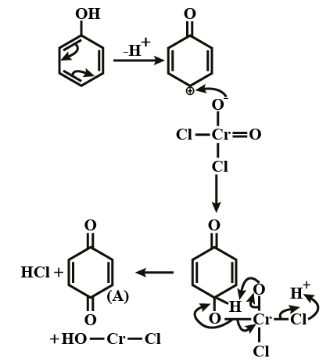
The reaction mechanism is shown below;
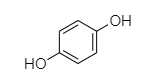
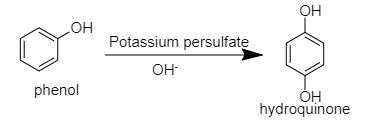
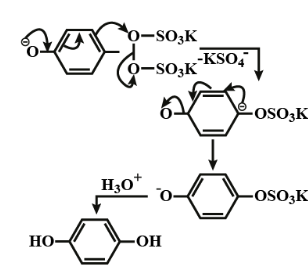
B. When phenol reacts with potassium persulfate then hydroquinone will be formed.
The reaction mechanism is shown below;
Additional Information:
-Chromyl chloride test is done for detecting the presence of ions of ionic compounds.
The reaction to the test is shown below:
${ K }_{ 2 }{ Cr }_{ 2 }{ O }_{ 7 }{ +{ 4Cl }^{ - }{ +6H }_{ 2 }{ SO }_{ 4 } }{ \rightarrow { 2CrO }_{ 2 }{ Cl } }_{ 2 }{ +2KHSO }_{ 4 }{ +4HSO }_{ 4 }^{ - }{ +3H }_{ 2 }{ O }$
-The physical properties of Chromyl chloride: Chromyl chloride is a dark red viscous fluid, which exhausts all around. It responds with water, alcohols, and is solvent in chlorinated carbons and carboxylic acids.
-It is used as an oxidizing agent.
-Safety considerations:
1.Acute: Exposure to chromyl chloride fume aggravates the respiratory framework and seriously bothers the eyes, and the fluid consumes the skin and eyes. Ingestion would cause serious interior harm.
2.Chronic: Cr(VI) can create chromosomal variations and is a human cancer-causing agent by means of inward breath. Visiting the presentation of the skin to chromyl chloride may bring about ulceration.
-Potassium persulfate: This salt is utilized to start polymerization of different alkenes prompting economically significant polymers, for example, styrene-butadiene elastic and polytetrafluoroethylene and related materials. In the arrangement, the dianion separates to give radicals.
Note:
The possibility of making a mistake is that chromyl chloride is an oxidizing agent, not a reducing agent, and is very dangerous as it is carcinogenic in nature. Be careful while using it.
Complete step by step answer:
When phenol reacts with chromyl chloride in presence of nascent oxygen then benzoquinone will be formed.

The reaction mechanism is shown below;

B. When phenol reacts with potassium persulfate then hydroquinone will be formed.

The reaction mechanism is shown below;

Additional Information:
-Chromyl chloride test is done for detecting the presence of ions of ionic compounds.
The reaction to the test is shown below:
${ K }_{ 2 }{ Cr }_{ 2 }{ O }_{ 7 }{ +{ 4Cl }^{ - }{ +6H }_{ 2 }{ SO }_{ 4 } }{ \rightarrow { 2CrO }_{ 2 }{ Cl } }_{ 2 }{ +2KHSO }_{ 4 }{ +4HSO }_{ 4 }^{ - }{ +3H }_{ 2 }{ O }$
-The physical properties of Chromyl chloride: Chromyl chloride is a dark red viscous fluid, which exhausts all around. It responds with water, alcohols, and is solvent in chlorinated carbons and carboxylic acids.
-It is used as an oxidizing agent.
-Safety considerations:
1.Acute: Exposure to chromyl chloride fume aggravates the respiratory framework and seriously bothers the eyes, and the fluid consumes the skin and eyes. Ingestion would cause serious interior harm.
2.Chronic: Cr(VI) can create chromosomal variations and is a human cancer-causing agent by means of inward breath. Visiting the presentation of the skin to chromyl chloride may bring about ulceration.
-Potassium persulfate: This salt is utilized to start polymerization of different alkenes prompting economically significant polymers, for example, styrene-butadiene elastic and polytetrafluoroethylene and related materials. In the arrangement, the dianion separates to give radicals.
Note:
The possibility of making a mistake is that chromyl chloride is an oxidizing agent, not a reducing agent, and is very dangerous as it is carcinogenic in nature. Be careful while using it.
Recently Updated Pages
If a wire of resistance R is stretched to double of class 12 physics JEE_Main

The path difference between two waves for constructive class 11 physics JEE_MAIN

What is the difference between solvation and hydra class 11 chemistry JEE_Main

IfFxdfrac1x2intlimits4xleft 4t22Ft rightdt then F4-class-12-maths-JEE_Main

Sodium chloride is purified by passing hydrogen chloride class 11 chemistry JEE_Main

Consider the following oxyanions PO43P2O62SO42MnO4CrO4S2O52S2O72 class 11 chemistry JEE_Main

Other Pages
The mole fraction of the solute in a 1 molal aqueous class 11 chemistry JEE_Main

If a wire of resistance R is stretched to double of class 12 physics JEE_Main

Lowering in vapour pressure is highest for A 02 m urea class 11 chemistry JEE_Main

An electric bulb has a power of 500W Express it in class 11 physics JEE_Main

Which of the following Compounds does not exhibit tautomerism class 11 chemistry JEE_Main

The ratio of speed of sound in Hydrogen to that in class 11 physics JEE_MAIN